Impact of an mHealth, Electronic Pillbox Intervention on Tacrolimus Variability in Kidney Transplantation: Results of a Prospective Randomized Clinical Trial
J. W. McGillicuddy, L. Sox, J. Chandler, F. Treiber, D. Taber
Medical University of South Carolina, Charleston, SC
Meeting: 2019 American Transplant Congress
Abstract number: D314
Keywords: Kidney transplantation, Outcome, Psychosocial
Session Information
Session Name: Poster Session D: Psychosocial and Treatment Adherence
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Medication non-adherence (MNA) is a major risk factor and leading cause of late allograft loss in kidney transplant (KTX) recipients. Tacrolimus trough concentration variability, measured using the coefficient of variation (Tac CV) is strongly correlated with graft outcomes, including acute rejection, graft function, and allograft loss. The objective of this study was to determine the effects of an mHealth intervention on tacrolimus trough concentration variability.
*Methods: This was a 6-month, prospective, parallel-arm, randomized controlled clinical trial. Patients randomized to the intervention arm utilized an electronic medication tray, Bluetooth-enabled BP monitor, and an mHealth app to monitor home-based adherence to their medical regimen. The control group participants received text messages containing healthy lifestyle tips for attention control. Tac variability was measured using a 12-month rolling average of the CV (std dev / mean), which was assessed at monthly intervals, starting at time of randomization and continuing for 12-months (6-months during the intervention period and 6-months after completion of the study). Statistical analysis was conducted using a generalized linear mixed model to account for correlation of repeated measures.
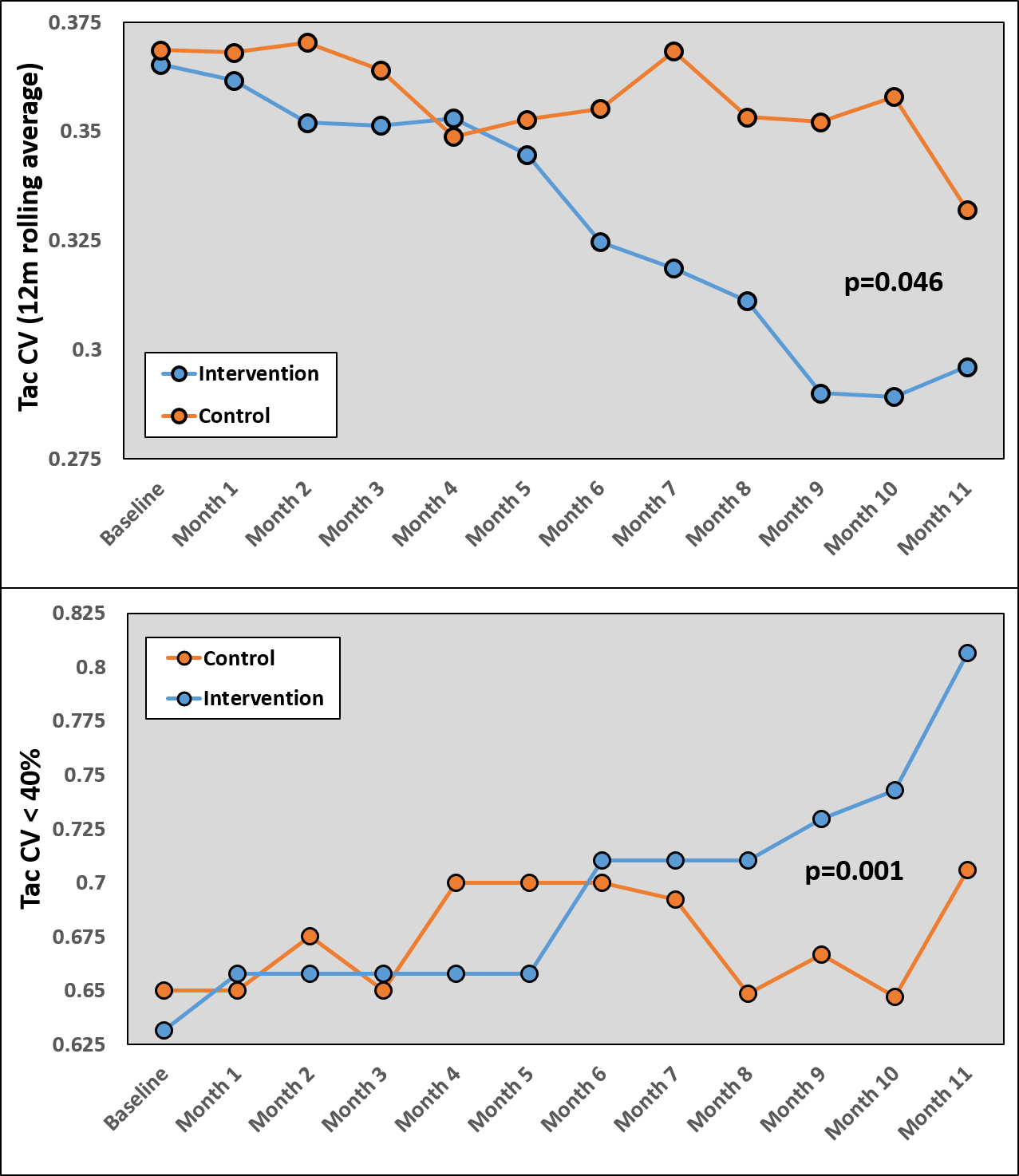
*Results: Eighty KTX recipients were enrolled in the study (40 in each arm). At time of randomization, participants mean age was 52±12 years, 32% were female, 77% were African-American, 9% received living donor organs, with a mean time post-txp of 2.0±2.2 years. These characteristics were similar between arms (p>0.5). At baseline, tacrolimus variability, as measured by the mean 12-month Tac CV, was similar between the intervention arm (36.9±14.5%) and the control arm (36.5±12.7%, p=0.894). Patients randomized into the intervention had a significant reduction in the mean 12-month rolling average of the tac CV (top Figure, p=0.046) and had a significant improvement in the proportion of patients achieving low tac variability (cut point tac CV <40%, bottom Fig, p=0.001), as compared to the control arm.
*Conclusions: This is the first clinical trial to demonstrate improved tacrolimus variability in KTX using an mHealth electronic pillbox intervention. As high tacrolimus variability is a well-studied risk factor for acute rejection and graft loss, these results offer promise to improve medication adherence and long-term clinical outcomes through an innovative methodology.
To cite this abstract in AMA style:
McGillicuddy JW, Sox L, Chandler J, Treiber F, Taber D. Impact of an mHealth, Electronic Pillbox Intervention on Tacrolimus Variability in Kidney Transplantation: Results of a Prospective Randomized Clinical Trial [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-an-mhealth-electronic-pillbox-intervention-on-tacrolimus-variability-in-kidney-transplantation-results-of-a-prospective-randomized-clinical-trial/. Accessed February 3, 2026.« Back to 2019 American Transplant Congress