Developing a Patient Reported Outcome Measure for Assessment of Calcineurin Inhibitor-Related Symptoms
J. L. Beaumont1, I. Purnajo1, M. Polinsky2, E. Alemao2, M. J. Everly1
1Terasaki Research Institute, Los Angeles, CA, 2Bristol Myers Squibb, Princeton, NJ
Meeting: 2019 American Transplant Congress
Abstract number: D304
Keywords: Quality of life
Session Information
Session Name: Poster Session D: Psychosocial and Treatment Adherence
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Calcineurin inhibitor (CNI) therapy has side effects which can potentially impact a patient’s health related quality of life (HRQoL). A standardized measure to assess side effects related to CNIs is needed to document profile differences between them and newer immunosuppressive agents. We used retrospectively reviewed symptoms data from the BENEFIT trial to develop a brief measure of side effects of CNI therapy, the calcineurin-inhibitor related symptom (CIRS) scale.
*Methods: From the BENEFIT trial (belatacept vs cyclosporine), 394 renal transplant patients were included in our analysis. All patients were asked to complete the MTSODS-59R (symptom occurrence/distress scales) at 12, 24, and 36 months post-transplant. Ridit analysis was used to compare the ordinal occurrence and distress ratings for each symptom between treatment arms at each time point. A CIRS score was calculated by summing distress scores for selected items. The CIRS score was compared between treatment arms, correlated with SF-36 HRQoL scores, and examined as a predictor of graft loss.
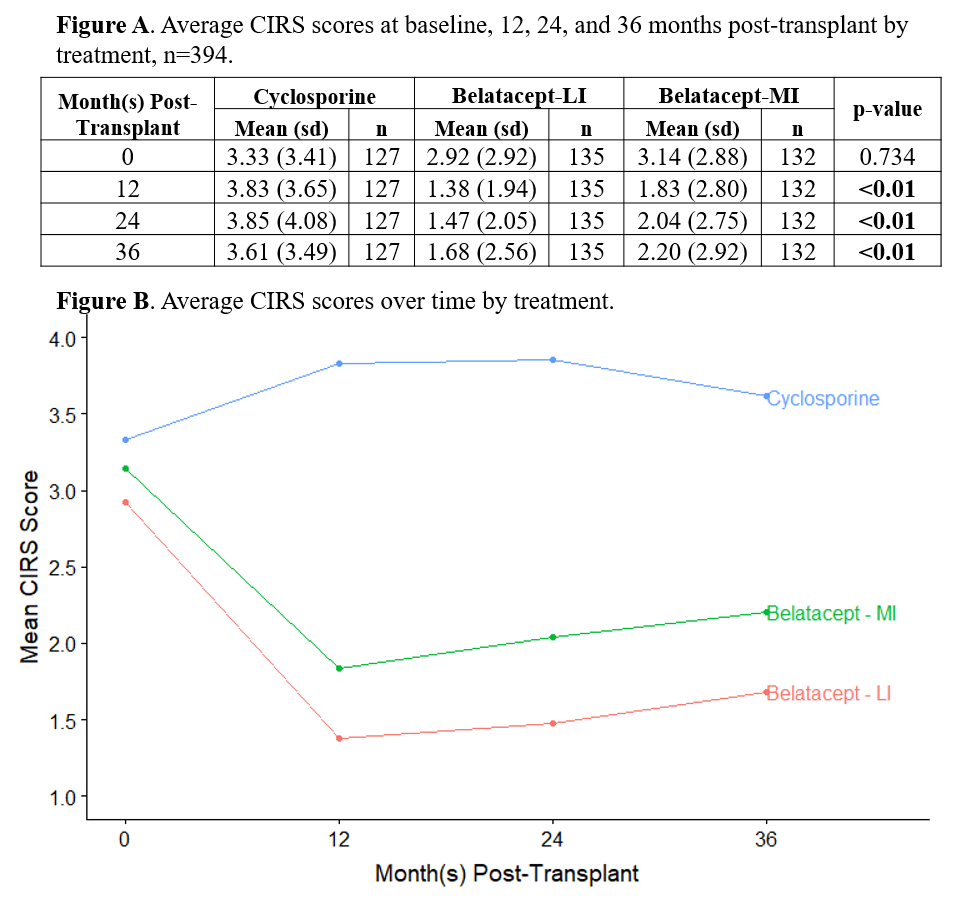
*Results: Symptoms that were both more frequent and more distressing in cyclosporine compared to belatacept-treated patients at all three post-transplant time points were trembling hands, muscle cramps, muscle weakness, swollen gums, and increased hair growth. At 12 months, the mean CIRS score (SD) was 3.83 (3.65) in the cyclosporine arm and 1.38 (1.94) in the belatacept-LI arm (p<0.01, Figure A, B). CIRS scores were significantly associated with all eight subscales of the SF-36, particularly bodily pain, vitality, physical function, social function, and mental health, but were not predictive of graft failure or survival.
*Conclusions: Cyclosporine is associated with a distinct set of symptoms that kidney transplant recipients report at higher levels of frequency and distress compared to patients treated with belatacept. Based on this preliminary evidence, a CIRS scale could be further developed and validated for use in research and clinical practice.
To cite this abstract in AMA style:
Beaumont JL, Purnajo I, Polinsky M, Alemao E, Everly MJ. Developing a Patient Reported Outcome Measure for Assessment of Calcineurin Inhibitor-Related Symptoms [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/developing-a-patient-reported-outcome-measure-for-assessment-of-calcineurin-inhibitor-related-symptoms/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress