Idarucizumab For The Reversal Of Dabigatran In Patients Undergoing Heart Transplantation
1Cardiology, University Hospitals Leuven, Leuven, Belgium, 2Anesthesiology, University Hospitals Leuven, Leuven, Belgium, 3Cardiovascular Surgery, University Hospitals Leuven, Leuven, Belgium, 4Nephrology, University Hospitals Leuven, Leuven, Belgium
Meeting: 2019 American Transplant Congress
Abstract number: B104
Keywords: Anticoagulation, Blood transfusion, Heart transplant patients, Surgical complications
Session Information
Session Name: Poster Session B: Heart and VADs: All Topics
Session Type: Poster Session
Date: Sunday, June 2, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Atrial fibrillation is common among patients with advanced heart failure who are listed for transplantation. Idarucizumab is a monoclonal antibody fragment that was developed to neutralize the activity of the direct thrombin inhibitor dabigatran. We describe the experience of dabigatran reversal with idarucizumab in 10 patients undergoing heart transplant surgery at the University Hospitals Leuven.
*Methods: At the time of listing for heart transplantation, patients requiring anticoagulation because of non-valvular atrial fibrillation, CHA2DS2VASc score ≥ 2 and without ventricular assist device or end-stage renal failure, were started on or switched to dabigatran. Upon availability of a donor organ, dabigatran was neutralized with 5 g of intravenous idarucizumab, immediately prior to induction of anaesthesia.
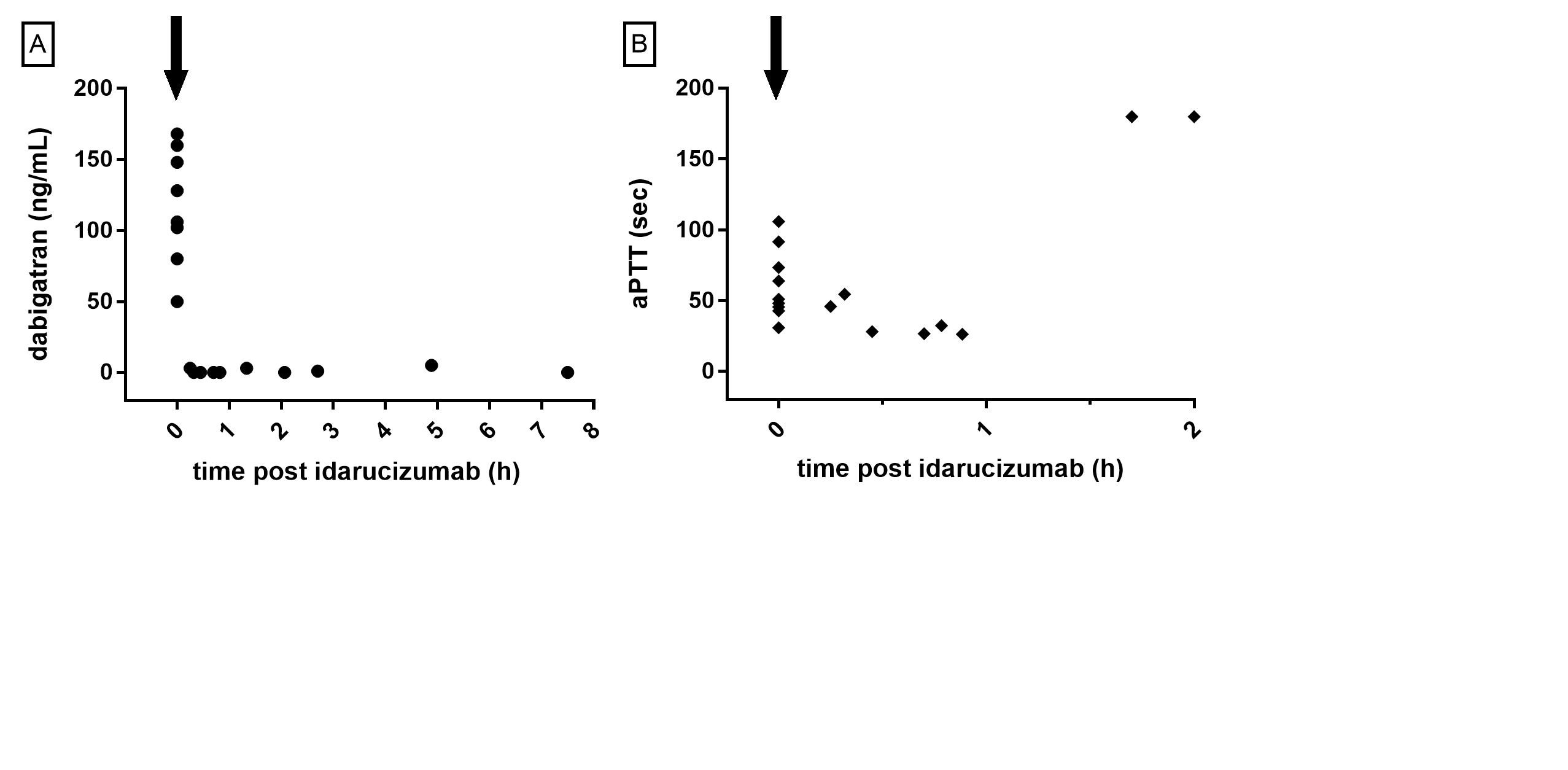
*Results: Ten patients have received a heart transplant using this protocol since its implementation at our centre on October 1st, 2015. Mean age was 57.9 years, 9 of the 10 patients were male, median CHA2DS2VASc score was 3 and mean eGFR at time of transplantation 53 mL/min. Mean time since last intake was 6.2 h. Evolution of dabigatran concentration (measured by a calibrated diluted thrombin time assay) and activated partial thromboplastin time (aPTT) in function of time after idarucizumab administration are represented in Figure 1. Mean dabigatran level before administration of idarucizumab was 117.8 ng/mL. All dabigatran concentrations post idarucizumab were unmeasurably low. Mean aPTT (reference range 25.1 – 36.5 s) was 55.8 s prior to idarucizumab and 35.4 s immediately post idarucizumab.
During surgery, patients received on average 1.0 unit of packed cell transfusion, 4.1 units fresh frozen plasma, 0.9 pools platelets and 587 mL blood that was recovered via cell salvage. Two patients (20.0 %) needed re-intervention because of bleeding. No adverse reactions or unexpected events that could potentially be related to idarucizumab administration were noted. There were no thrombotic complications.
*Conclusions: This is the largest report describing the use of idarucizumab to normalize coagulation in patients on dabigatran awaiting heart transplantation. Administration of 5 g of idarucizumab led to a sustained and complete biochemical reversal, without thrombotic complications, and without interfering with heparinization for cardiopulmonary bypass. However, there still were some bleeding events. The availability of an immediately-acting complete reversal agent makes dabigatran an attractive choice for non-VAD patients with non-valvular atrial fibrillation who are listed for heart transplantation.
To cite this abstract in AMA style:
Keer JVan, Vanassche T, Droogne W, Rex S, Rega F, Cleemput JVan, Verhamme P, Naesens M. Idarucizumab For The Reversal Of Dabigatran In Patients Undergoing Heart Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/idarucizumab-for-the-reversal-of-dabigatran-in-patients-undergoing-heart-transplantation/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress