Clinical Implications of Belatacept Conversion in a High-Risk, Urban Renal Transplant Population
University of Illinois at Chicago, Chicago, IL
Meeting: 2019 American Transplant Congress
Abstract number: A244
Keywords: Co-stimulation, Immunosuppression, Kidney, Nephrotoxicity
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Conversion to belatacept as maintenance immunosuppression has gained traction to prevent calcineurin inhibitor (CNI) induced toxicities in renal transplant (RTx) recipients. However, limited knowledge exists regarding the clinical impact of this strategy on high-risk, obese, and racially diverse patients. The purpose of this study was to compare renal, cardiovascular, and metabolic health outcomes pre- and post-belatacept conversion in a high-risk, urban RTx population.
*Methods: This was a retrospective cohort study of high-risk patients who underwent RTx from 1/1/2012-12/31/2017 and were converted to belatacept. High-risk RTx was defined as: African American race, panel reactive antibody > 10%, re-transplant, or positive flow-crossmatch. The primary outcome was to compare estimated glomerular filtration rate (eGFR) at time of belatacept conversion to 6 months post-conversion. Secondary outcomes included incidence of biopsy-proven acute rejection (BPAR) post-conversion, and comparisons of cardiovascular, metabolic, and infectious outcomes. Obesity and conversion timing (early conversion < 90 days post-RTx) were assessed to determine impact on percent change in eGFR (%eGFR) pre- versus 6 months post-conversion. Data were compared with McNeamer's test, paired t-test, and Wilcoxon signed ranks test.
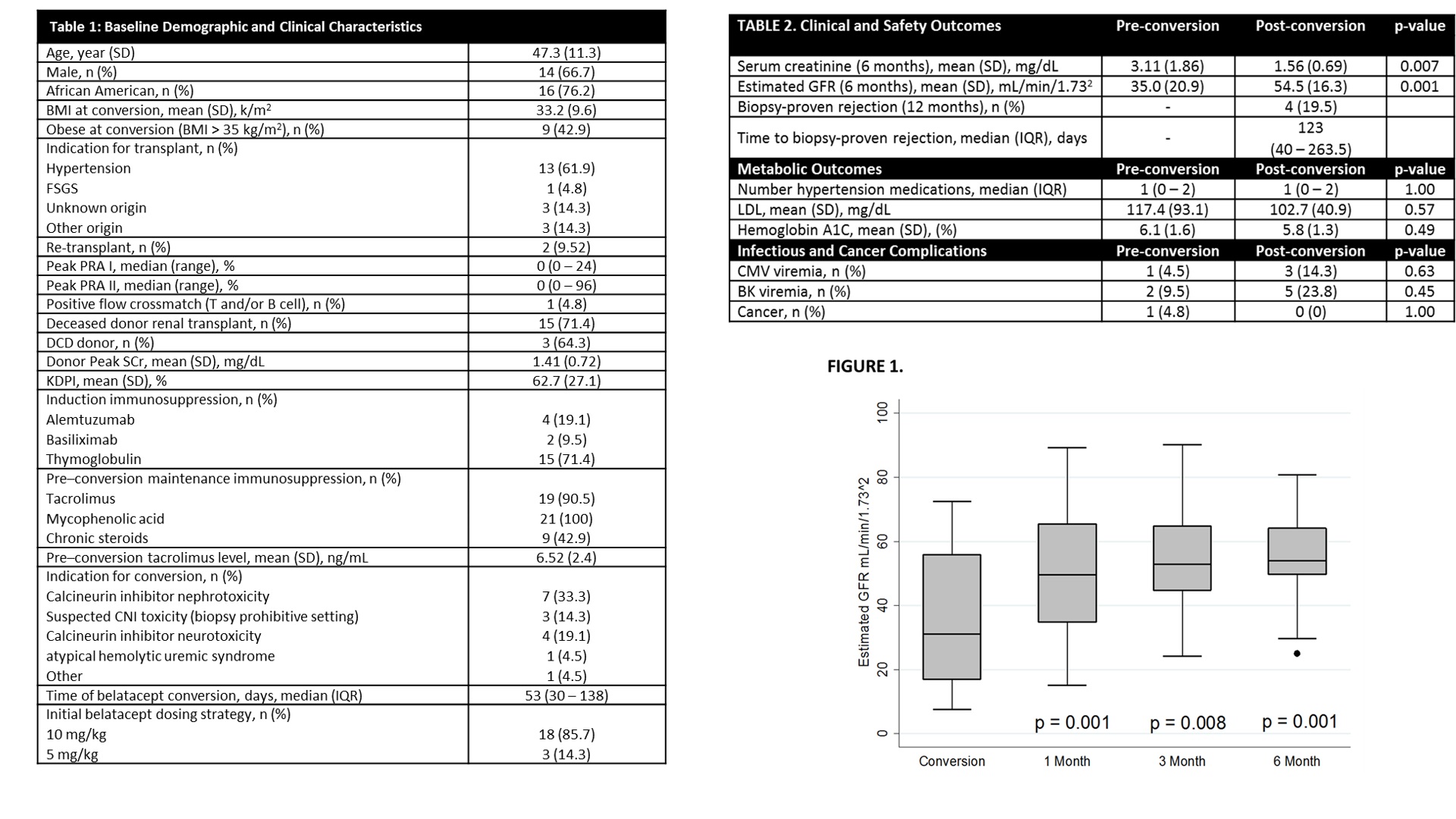
*Results: 21 RTx recipients were analyzed. A majority of patients were male (66.7%), African American (76.2%) undergoing belatacept conversion with a starting dose of 10mg/kg (85.7%) at a median of 53 days post-RTx. CNI nephrotoxicity (47.6%) was the major conversion indication. Table 1 details demographic information. Estimated GFR improved significantly (pre-conversion 35.0 mL/min/1.732 vs. 6 months post-conversion 54.5 mL/min/1.732, p=0.001). Four patients (19.5%) experienced a BPAR at a median of 123 (IQR 40 – 263.5) days post-conversion. Despite this finding, eGFR was higher after belatacept conversion at all time points (Figure 1). Obese patients experienced a larger numeric improvement in %eGFR compared to non-obese patients (68.4% vs 20.7%, p = 0.143). Late conversion did not impact %eGFR (p=0.379). No differences in cardiovascular, metabolic, and infectious outcomes post-conversion were observed (Table 2).
*Conclusions: Belatacept conversion can be accomplished in a high-risk population. BPAR is of concern, but allograft function improvement is evident as early as 1 month post-conversion. Larger studies examining risks for post-conversion rejection and allograft response are essential to identifying the best method in this complex population.
To cite this abstract in AMA style:
Valdepenas B, Benken J, Benedetti E, Hajjiri Z, Lichvar A. Clinical Implications of Belatacept Conversion in a High-Risk, Urban Renal Transplant Population [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-implications-of-belatacept-conversion-in-a-high-risk-urban-renal-transplant-population/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress