Dosing and Exposure over 2 Years with LCP-Tacrolimus (LCPT) vs. Immediate-Release Tacrolimus (IR-Tac) in De Novo Kidney Transplantation
1Charité University Medicine Berlin, Berlin, Germany, 2UCLA Medical Center, Los Angeles, CA, 3Veloxis Pharmaceuticals Inc, Cary, NC, 4University Hospital of Münster, Münster, Germany
Meeting: 2019 American Transplant Congress
Abstract number: A238
Keywords: FK506, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
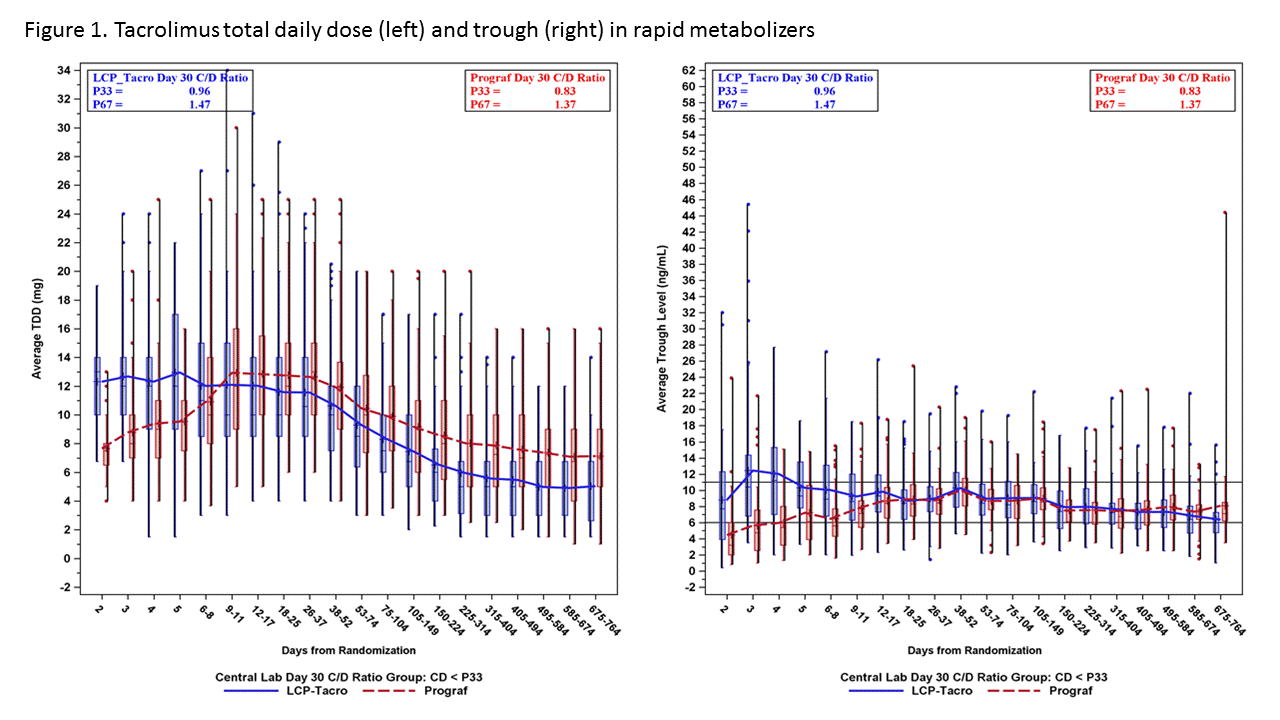
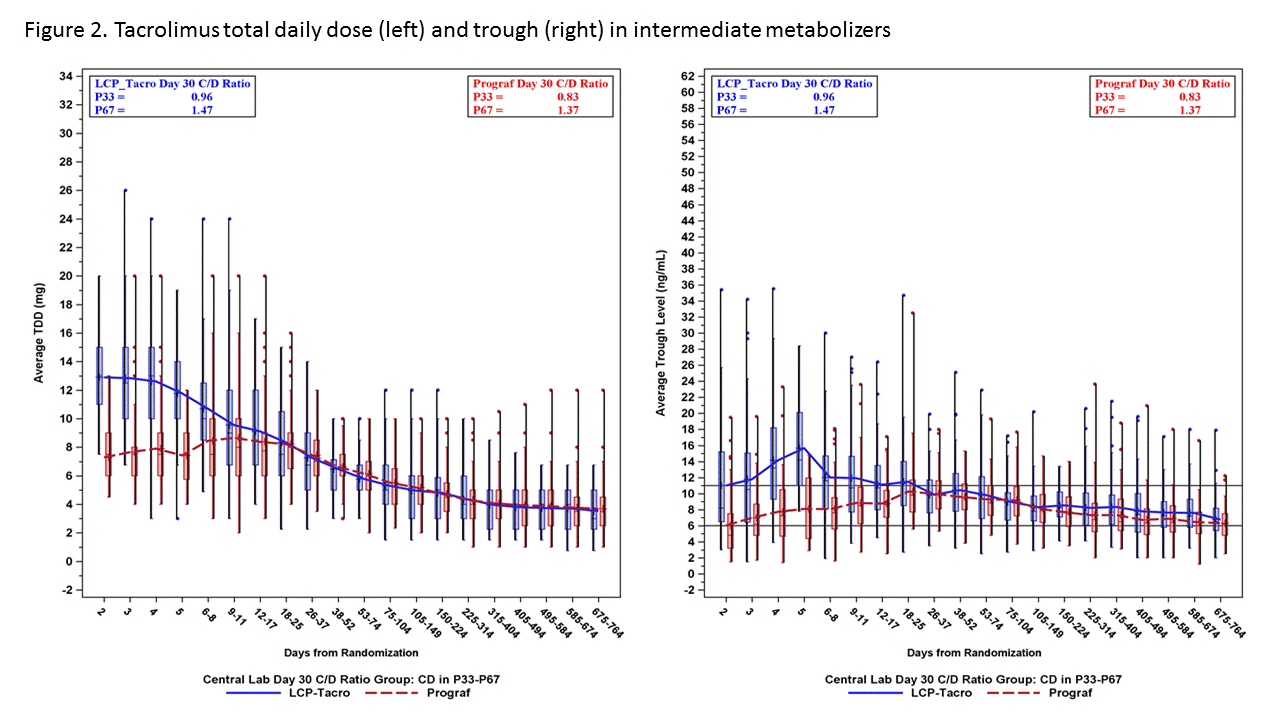
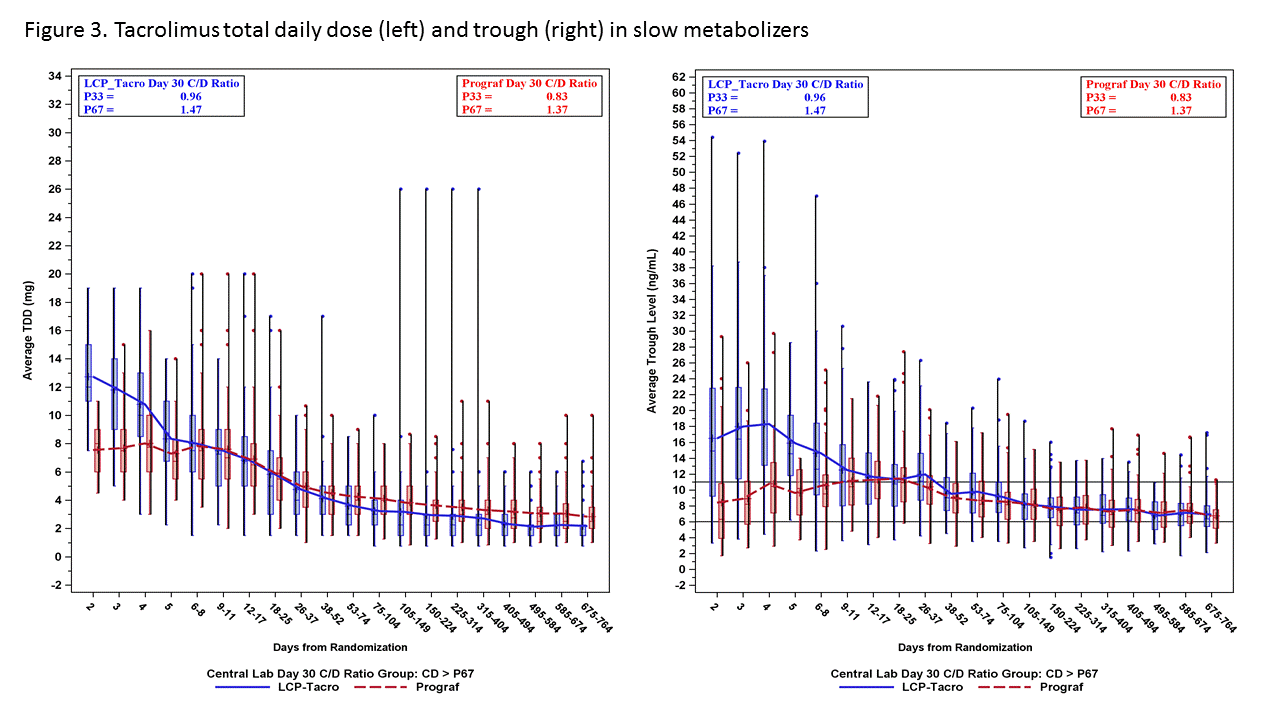
*Purpose: A prospective study demonstrated safe and efficacious use of LCPT in de novo kidney transplantation. This study aims to evaluate tacrolimus trough levels and total daily dosing (TDD) over time in patients on LCPT vs. IR-Tac based on tacrolimus metabolism rate.
*Methods: A post-hoc analysis of a Phase III trial was conducted. Within 48 hours of transplant, patients were randomized to receive LCPT 0.17 mg/kg/day or IR-Tac 0.1 mg/kg/day. Subsequent doses were adjusted to target trough levels of 6-11 ng/mL for the first 30 days, then 4-11 ng/mL. Patients were divided into low, medium and high metabolizer groups based on Day 30 concentration-to-dose (CD) ratio tertiles.
*Results: A total of 543 patients were included for evaluation. Rapid metabolizers (lowest CD ratio tertile; figure 1) demonstrated relatively stable TDD over the first 4 weeks with resultant tacrolimus trough levels at or just above the target range as early as day 2. In the same cohort, IR-Tac treated patients required increases in TDD to reach a mean trough within target range. In contrast, intermediate and slow metabolizers demonstrated mean trough levels above target with LCPT dosed at 0.17 mg/kg, requiring dose decreases over time (Figures 2 and 3).
*Conclusions: These data suggest that an LCPT dose of 0.17 mg/kg/day results in hastened achievement of therapeutic tacrolimus levels in rapid metabolizers and minimal change in average total daily dosing early post-transplant. A lower starting dose for LCPT may be more appropriate for slow and intermediate metabolizers as data indicate average trough concentrations above target and more rapid downward dose titration in early days post-transplant.
To cite this abstract in AMA style:
Budde K, Bunnapradist S, Patel S, Stevens D, Meier-Kriesche U, Suwelack B. Dosing and Exposure over 2 Years with LCP-Tacrolimus (LCPT) vs. Immediate-Release Tacrolimus (IR-Tac) in De Novo Kidney Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/dosing-and-exposure-over-2-years-with-lcp-tacrolimus-lcpt-vs-immediate-release-tacrolimus-ir-tac-in-de-novo-kidney-transplantation/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress