Post-Kidney Transplant Infections in Desensitized Patients Receiving Thymoglobulin or Daclizumab Induction: Results of a Randomized Clinical Trial
1Department of Surgery, Johns Hopkins University, Baltimore
2Department of Surgery, University of Alabama--Birmingham, Birmingham
3Department of Medicine, Johns Hopkins University, Baltimore
4Department of Surgery, Dartmouth, Lebanon.
Meeting: 2015 American Transplant Congress
Abstract number: 173
Keywords: Highly-sensitized, Immunosuppression, Infection, Kidney transplantation
Session Information
Session Name: Concurrent Session: ID - Epidemiology, Resistance, Geographic Infections
Session Type: Concurrent Session
Date: Monday, May 4, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:39pm-2:51pm
Presentation Time: 2:39pm-2:51pm
Location: Room 115-C
Live donor kidney transplant recipients requiring desensitization for donor-specific antibody, who were enrolled in a single-center randomized trial of thymoglobulin vs. daclizumab induction, were evaluated for infectious complications in the first year post-transplant using an intent-to-treat analysis. Fifty-six patients were randomized, 30 to the thymoglobulin arm and 26 to daclizumab. All patients received plasmapheresis and low dose CMVIg (100 mg/kg). The overall incidence of serious infections in the first year was high (85.7%; 83.3 in the thymoglobulin arm vs. 88.5% daclizumab arm; P=0.58; for infections other than UTI's, 63.3% vs. 65.4%, p=0.87).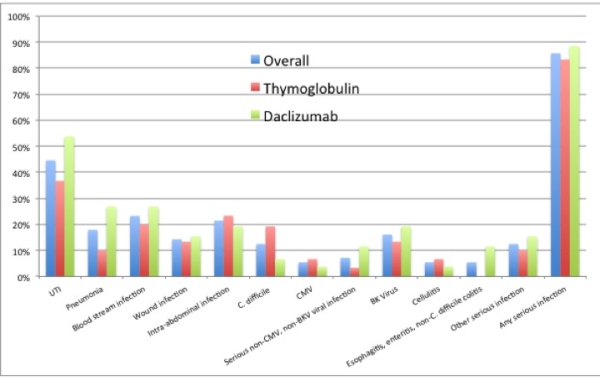 Two patients, both in the daclizumab arm, died from infection (one from RSV pneumonia and one from H1N1 influenza). While there was no difference between the two groups in terms of UTI/pyelonephritis, bloodstream infections, wound infections, intra-abdominal infections, C. difficile, CMV, BK virus, or cellulitis, , there was a trend towards higher incidence of pneumonia (26.9% vs 10.0%; P=0.10) and GI infection other than C. difficile (11.5% vs. 0%; P=0.06) in the daclizumab group (Figure 1). The daclizumab arm also had a higher per-patient incidence of serious infections the first year (2.5 versus 1.6; P=0.04). In conclusion, the majority of desensitized patients in a randomized trial of thymoglobulin vs. daclizumab induction developed serious infections in the first year post-transplant, with higher per-patient incidence of total infections, and trends toward higher incidence of certain specific infections in the daclizumab group. These results should be confirmed in larger studies, but suggest that immunologically high-risk patients are not at lower risk of infection when receiving daclizumab induction.
Two patients, both in the daclizumab arm, died from infection (one from RSV pneumonia and one from H1N1 influenza). While there was no difference between the two groups in terms of UTI/pyelonephritis, bloodstream infections, wound infections, intra-abdominal infections, C. difficile, CMV, BK virus, or cellulitis, , there was a trend towards higher incidence of pneumonia (26.9% vs 10.0%; P=0.10) and GI infection other than C. difficile (11.5% vs. 0%; P=0.06) in the daclizumab group (Figure 1). The daclizumab arm also had a higher per-patient incidence of serious infections the first year (2.5 versus 1.6; P=0.04). In conclusion, the majority of desensitized patients in a randomized trial of thymoglobulin vs. daclizumab induction developed serious infections in the first year post-transplant, with higher per-patient incidence of total infections, and trends toward higher incidence of certain specific infections in the daclizumab group. These results should be confirmed in larger studies, but suggest that immunologically high-risk patients are not at lower risk of infection when receiving daclizumab induction.
To cite this abstract in AMA style:
Orandi B, Locke J, Kraus E, Lonze B, Desai N, Dagher N, Alachkar N, Simpkins C, Naqvi F, Segev D, Montgomery R, Avery R. Post-Kidney Transplant Infections in Desensitized Patients Receiving Thymoglobulin or Daclizumab Induction: Results of a Randomized Clinical Trial [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/post-kidney-transplant-infections-in-desensitized-patients-receiving-thymoglobulin-or-daclizumab-induction-results-of-a-randomized-clinical-trial/. Accessed January 6, 2026.« Back to 2015 American Transplant Congress
