Prospective, Iterative, Phased, Trial of Proteasome Inhibitor-Based Desensitization in Highly Sensitized Renal Transplant Candidates, A
U of Cincinnati, Cincinnati, OH
Hoxworth Blood Center, Cincinnati
Hoxworth Blood Center, Cincinnati
The Christ Hospital, Cincinnati
Meeting: 2013 American Transplant Congress
Abstract number: 151
Substantive prospective trials of proteasome inhibitor (PI)-based desensitization (DS) have not been reported. This study prospectively evaluated PI-based DS in highly HLA sensitized kidney transplant (KTx) candidates under an FDA IND.
METHODS: Study included 5 phases, which were iteratively designed with prior phases instructing subsequent phase design. Phases included 1 or 2 bortezomib (BTZ) cycles (1.3 mg/m2 x 6-8 doses), single rituximab dose (375 mg/m2, max 500mg), and plasmapheresis. Phases differed in BTZ dosing density and plasmapheresis timing. DSA were measured by single antigen beads (SAB); dilutional analysis performed for SAB saturation. Immunodominant antibody (iAb) was defined as HLA Ab with highest MFI.
RESULTS: 44 patients (pts) received 52 DS courses: Phase 1 (n=20), 2 (n=12), Phase 3 (n=10), Phase 4 (n=5), Phase 5 (n=5). Statistically significant iAb reductions occurred in each phase with 38 of 44 (86%) pts showing iAb reduction. In Phase I, a 30.4% iAb reduction was observed at 30days with BTZ alone. HLA Abs at moderate (4000-8000 MFI) and low (1500-3000 MFI) levels showed greater reductions than iAbs. iAb reductions were observed for Class I and II iAbs, and for public antigens (DR 51, 52, 53). iAb reductions increased progressively in successive phases as BTZ dosing density increased. Ab levels remained suppressed for several weeks after treatment. T cell flow crossmatch decreased in 11/12 (91.5%) of pts with live donors (LDs) by mean of 103±54 MCS (log scale). 15 of 44 pts underwent KTx: 8 with LDs; 7 with deceased donors; acute rejection rate was 21%. Figure presents phase 3-5 iAb levels; arrows depict final DS treatment.
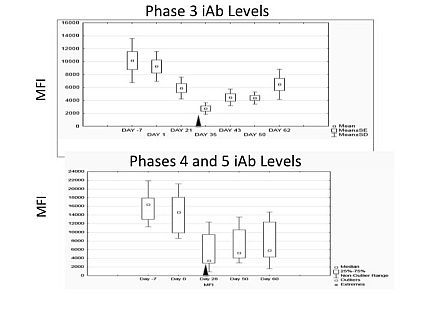
CONCLUSIONS: PI-based DS provides substantial reductions in HLA Ab levels in 85% of pts and persist for up to several weeks allowing for increased transplantability. These data support development of second generation plasma cell targeting DS regimens.
Woodle, E.: Grant/Research Support, Millennium.
To cite this abstract in AMA style:
Woodle E, Sadaka B, Walsh R, Shields A, Girnita A, Brailey P, Alloway R, Cardi M, Govil A, Jawdeh BAbu, Chaudhury PRoy, Mogilishetty G. Prospective, Iterative, Phased, Trial of Proteasome Inhibitor-Based Desensitization in Highly Sensitized Renal Transplant Candidates, A [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/prospective-iterative-phased-trial-of-proteasome-inhibitor-based-desensitization-in-highly-sensitized-renal-transplant-candidates-a/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
