Impact of Once-Daily Tacrolimus on Trough Concentration Variability in Stable Adolescent and Young Adult Renal Transplant Recipients
Children's Hospital Colorado, Aurora, CO
Meeting: 2019 American Transplant Congress
Abstract number: 528
Keywords: FK506, Immunosuppression, Kidney transplantation
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Novel Regimens and Drug Minimization III
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Veterans Auditorium
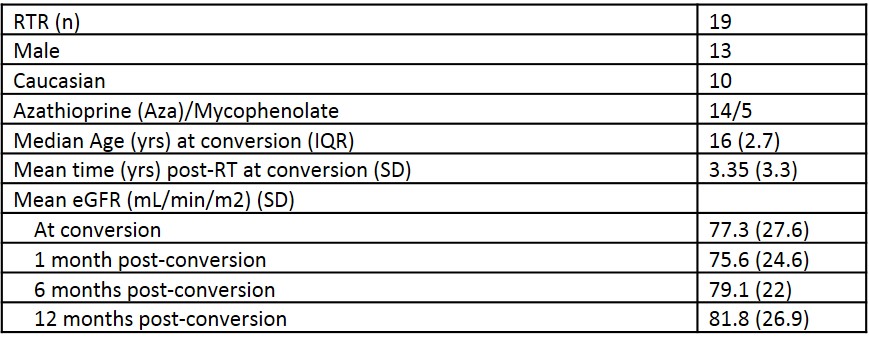
*Purpose: High tacrolimus concentration variability (Vtac), likely influenced by medication non-adherence, is a risk factor for graft dysfunction and loss among renal transplant recipients (RTRs), especially adolescents and young adults (AYA). Number and frequency of medication doses per day is a risk factor known to impact adherence. We evaluated the impact of conversion from twice-daily tacrolimus (Tac) to once-daily sustained-release Tac (srTac, Astagraf®) in AYA RTRs on medication adherence (assessed by Vtac).
*Methods: We studied 19 stable AYA RTRs (≥13 y) converted from twice-daily Tac to once-daily srTac. Maintenance immunosuppression consisted of Tac, antiproliferative and steroids. Adherence was assessed by measuring Vtac pre- and post-conversion (1, 6, and 12 months following conversion), and compared to pre-transplant adherence as measured by Pediatric Transplant Rating Instrument (PTRI).
*Results: In stable AYA RTRs, Vtac improved following conversion to srTac as evidenced by a narrowing standard deviation of Vtac at 30 days post-conversion (2.7 (1.4) vs. 1.2 (0.8), p = 0.001). Improvement persisted 6-months (1.2 (0.78), p = 0.007) and 12-months (0.8 (0.67), p = 0.001) following conversion. Post-conversion trough concentrations remained lower than those observed with twice-daily Tac (at 12 months: 5.2 (1.4) ng/mL vs. 6.9 (2.1) ng/mL, p = 0.023). To achieve target Tac concentrations, a median dose conversion of 1:1.25 was required. Following conversion to srTac, pill burden was reduced by 18% (11.8 (3.6) vs. 9.7 (3.54), p = 0.01). The majority of patients converted to srTac received Aza (73.7%), consistent with our goal of offering once-daily immunosuppression. As well, 47.4% of patients received once daily formulations for all medications. Renal function remained stable 1 year following conversion (eGFR 82 (27) vs. 79 (22) =0.74), and only one patient (5.3%) experienced acute cellular rejection. There was no difference in prevalence of EBV, CMV and BK viremias before and after conversion. Pre-transplant PTRI scoring was not associated with Vtac variability pre or post-conversion to srTAC (p: 0.9).
*Conclusions: We conclude that Vtac is improved with conversion to srTac, in combination with Aza, in AYA RTRs. Therefore, in this at-risk population, once daily dosing appears to improve adherence. Furthermore, such conversion does not appear to put graft function at risk. Identifying these at-risk AYAs for such intervention remains challenging with screening tools and clinical evaluation, necessitating the importance of ongoing multimodal assessment.
To cite this abstract in AMA style:
Moss M, Goebel J, Lofton A, Steinberg E, Bock M. Impact of Once-Daily Tacrolimus on Trough Concentration Variability in Stable Adolescent and Young Adult Renal Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/impact-of-once-daily-tacrolimus-on-trough-concentration-variability-in-stable-adolescent-and-young-adult-renal-transplant-recipients/. Accessed February 18, 2026.« Back to 2019 American Transplant Congress