LCP-Tacrolimus (LCPT; Envarsus XR) Dosing Considerations in De Novo Kidney Transplant Recipients
1University of Kansas Medical Center, Kansas City, KS, 2University of Illinois Hospital and Health Sciences System, Chicago, Chicago, IL, 3Veloxis Pharmaceuticals, Cary, NC, 4Veloxis Pharmaceuticals Inc., Cary, NC
Meeting: 2019 American Transplant Congress
Abstract number: 527
Keywords: FK506, Immunosuppression, Kidney transplantation
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Novel Regimens and Drug Minimization III
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Veterans Auditorium
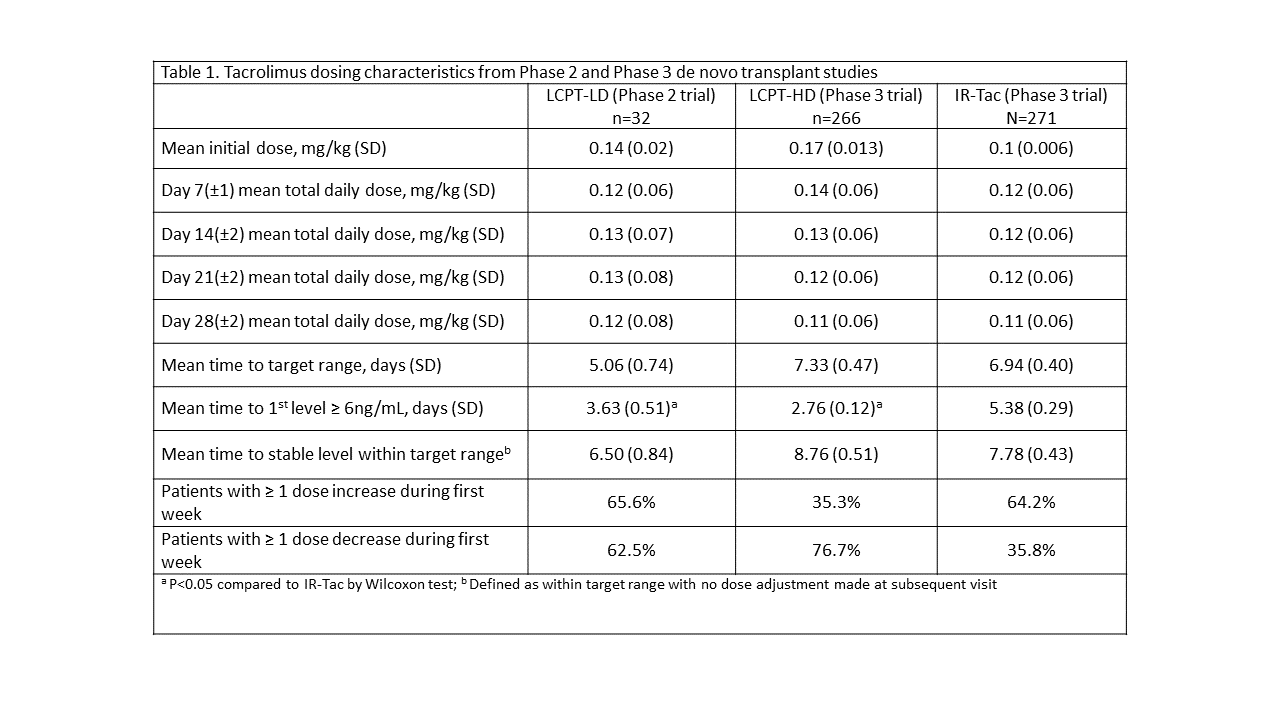
*Purpose: Different initial dosing strategies were studied in Phase II/Phase III trials evaluating the pharmacokinetics, safety, and efficacy of LCPT in de novo kidney transplantation. The purpose of this analysis is to describe patterns in early tacrolimus trough concentrations at these doses versus those seen with twice daily immediate-release tacrolimus (IR-Tac).
*Methods: A post-hoc analysis of studies comparing LCPT to IR-Tac was conducted. LCPT was initiated at 0.14 (lower dose or “LCPT-LD”) or 0.17 mg/kg/day (higher dose, or LCPT-HD), while IR-Tac was initiated at 0.1 mg/kg/day, divided. Tacrolimus was initiated within 48 hours of transplant. Time to target range (defined as tacrolimus concentrations of 6 – 11 ng/mL), percent of patients within target range, and frequency of dose changes during the first weeks post-transplant are reported.
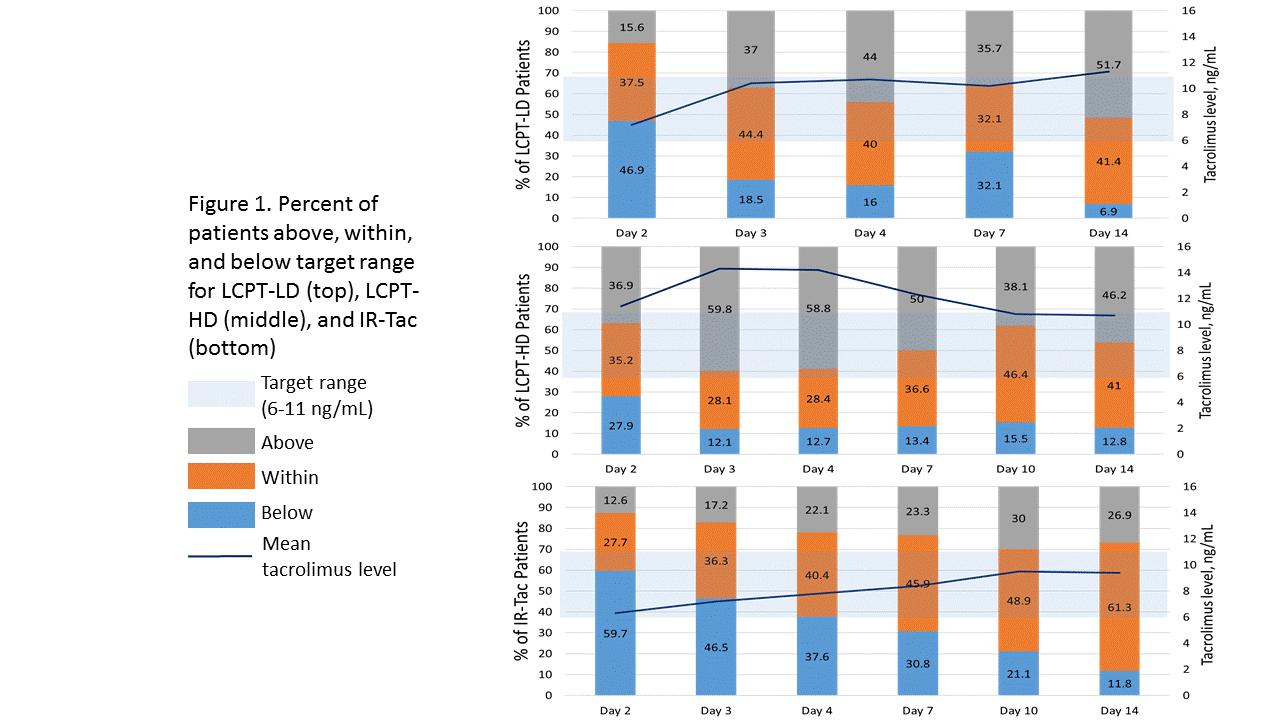
*Results: 569 de novo transplant recipients are included in the analysis (LCPT, n=298; IR-tac, n=271). Fewer patients received LCPT-LD due to the smaller, Phase II nature of the study. Tacrolimus dosing characteristics are shown in Table 1. Time to target level was lowest among patients initiated on LCPT-LD (5.06 days) and both LCPT groups exhibited significantly shorter time to obtain a tacrolimus concentration of at least 6 ng/mL compared to IR-Tac. More LCPT-LD patients remained within the target range during the first two weeks compared to the LCPT-HD patients, whereas more LCPT-HD patients reached trough concentrations above 11 ng/mL compared to LCPT-LD or IR-Tac immediately after transplant (Figure 1).
*Conclusions: LCPT-HD resulted in fewer patients with subtherapeutic tacrolimus concentrations early post-transplant but more patients with levels >11 ng/mL vs IR-Tac. LCPT-LD may represent a feasible initial dosing strategy that allows for rapid attainment of tacrolimus blood concentrations ≥6 ng/mL while exposing fewer patients to early trough concentrations >11 ng/mL versus LCPT-HD, though further evaluation in larger patient populations is needed to confirm this finding.
To cite this abstract in AMA style:
Cibrik D, West-Thielke P, Patel SJ, Stevens DR, Meier-Kriesche U. LCP-Tacrolimus (LCPT; Envarsus XR) Dosing Considerations in De Novo Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/lcp-tacrolimus-lcpt-envarsus-xr-dosing-considerations-in-de-novo-kidney-transplant-recipients/. Accessed February 19, 2026.« Back to 2019 American Transplant Congress