Patient-Reported Transplant-Related Symptoms in Kidney Transplant Recipients
J. L. Beaumont1, I. Purnajo1, M. Polinsky2, E. Alemao2, M. J. Everly1
1Terasaki Research Institute, Los Angeles, CA, 2Bristol Myers Squibb, Princeton, NJ
Meeting: 2019 American Transplant Congress
Abstract number: 183
Keywords: Quality of life
Session Information
Session Name: Concurrent Session: Psychosocial and Treatment Adherence
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Room 208
*Purpose: Kidney transplant (txp) recipients experience many symptoms as a result of their transplant experience, immunosuppressive regimens, and comorbid conditions. The objective of this analysis was to evaluate a transplant-related symptoms (TRS) score consisting of those patient-reported symptoms that have the strongest association with general health related quality of life (HRQoL) post-txp.
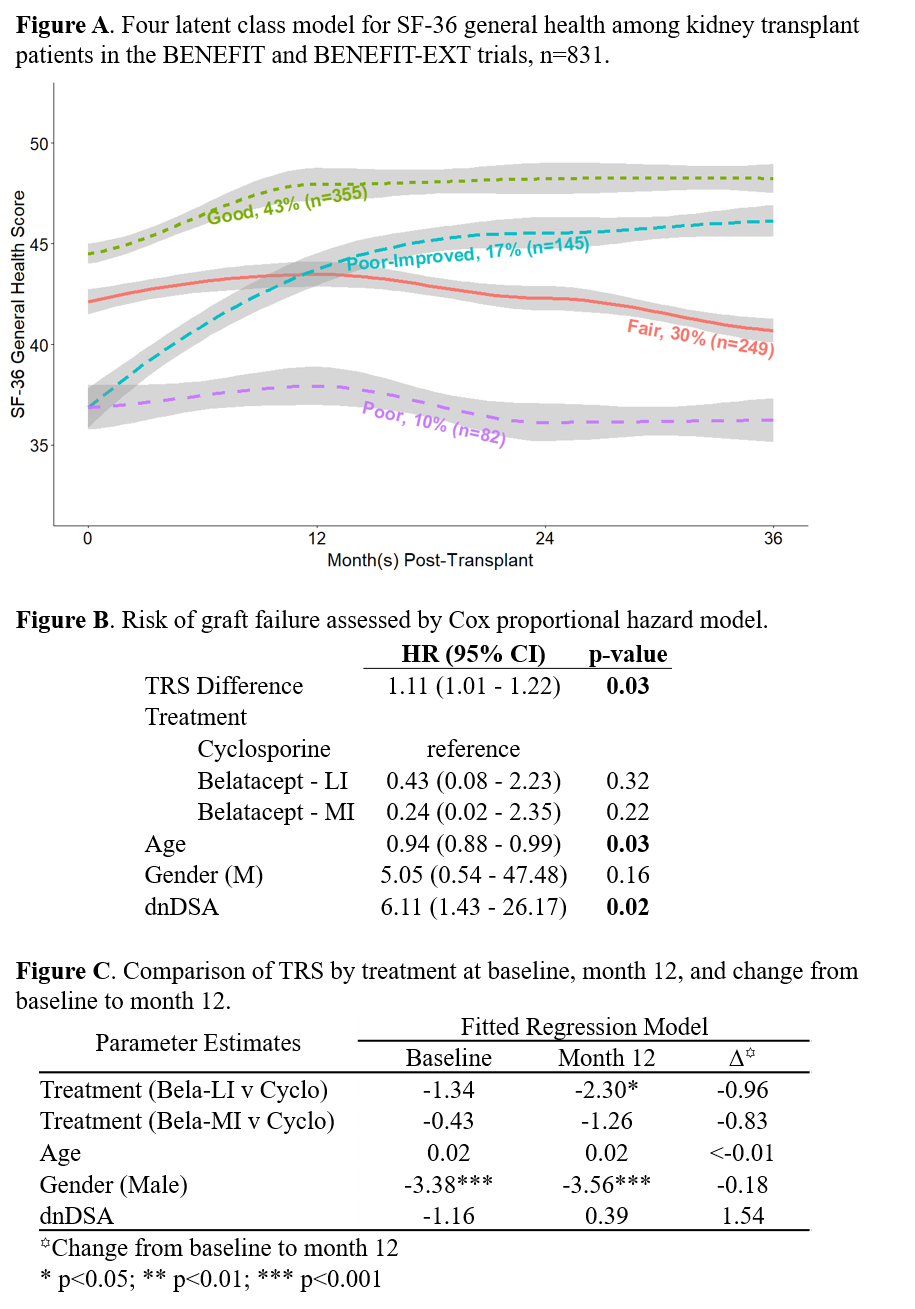
*Methods: In the BENEFIT trial (belatacept vs. cyclosporine), kidney txp recipients were assessed via the SF-36 (HRQoL) and MTSODS-59R (symptoms) at baseline, 12, 24, and 36 months post-txp. Latent class analysis was used to identify distinct trajectories of SF-36 general health scores over time (Figure A). Baseline symptom ratings and changes in symptom ratings from baseline to month 12 were examined for associations with general health trajectory. A TRS score was calculated by summing distress scores for selected items; this score was compared between treatment arms to demonstrate its potential utility as an endpoint, and examined as a predictor of txp outcomes.
*Results: After excluding patients with missing MTSODS-59R data, graft failure within the first year, and drug discontinuation within the first year, 394 patients were included in the analysis. Most patients grouped into one of three relatively stable trajectories defined by level of general health at baseline (similar to, 0.5 SD worse, or 2.0 SD worse than the general population) but a 4th group began 2SD below the general population mean and improved dramatically between transplant and 1 year (Figure A). The TRS score (Cronbach’s alpha=0.86) calculated from these items at baseline was not associated with graft failure but TRS change from baseline to month 12 was (HR: 1.11, 95% CI: 1.01-1.22, p=0.03, Figure B). Neither baseline TRS or change from baseline to month 12 differed by treatment arm. However, TRS at month 12 differed by treatment, particularly for patients receiving the belatacept LI (less intense) regimen (β = -2.30, p = 0.02, Figure C), when compared to cyclosporine.
*Conclusions: Based on this preliminary evidence using items of the MTSODS-59R, a TRS scale could be developed and validated for use in the assessment of transplant symptoms in research and clinical practice.
To cite this abstract in AMA style:
Beaumont JL, Purnajo I, Polinsky M, Alemao E, Everly MJ. Patient-Reported Transplant-Related Symptoms in Kidney Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/patient-reported-transplant-related-symptoms-in-kidney-transplant-recipients/. Accessed February 9, 2026.« Back to 2019 American Transplant Congress