Hope Act Donors With Organs Recovered For Transplant: The First Two Years
W. A. Werbel, O. Kusemiju, B. Barnaba, M. Bowring, B. Doby, C. Kirby, R. Fernandez, Y. Eby, J. Miller, D. Brown, S. Seaman, D. Ostrander, A. Tobian, D. Segev, C. M. Durand
Johns Hopkins University, Baltimore, MD
Meeting: 2019 American Transplant Congress
Abstract number: 21
Keywords: Donors, unrelated, HIV virus, Risk factors
Session Information
Session Name: Concurrent Session: Kidney Donor Selection / Management Issues I
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Ballroom C
*Purpose: The HOPE Act expands the potential deceased donor pool to include HIV+ donors (HIV D+) and donors with false-positive (FP) HIV screens. The objective of our study was to characterize the epidemiology and biology of HOPE Act donors who had organs recovered for transplant to HIV+ recipients (R+).
*Methods: Within the ongoing multicenter HOPE in Action trial (NCT02602262) investigating safety and outcomes of HIV D+/R+ transplantation, we collected demographic and clinical data from HIV-uninfected (D-), HIV D+ and FP donors who had organs recovered for transplant for HIV R+ recipients. Factors were compared via chi-squared, Fisher’s exact, ANOVA and Kruskal-Wallis tests. For D+, we tested blood samples for CD4 count, HIV viral load, cellular tropism and presence of antiretroviral (ART) resistance.
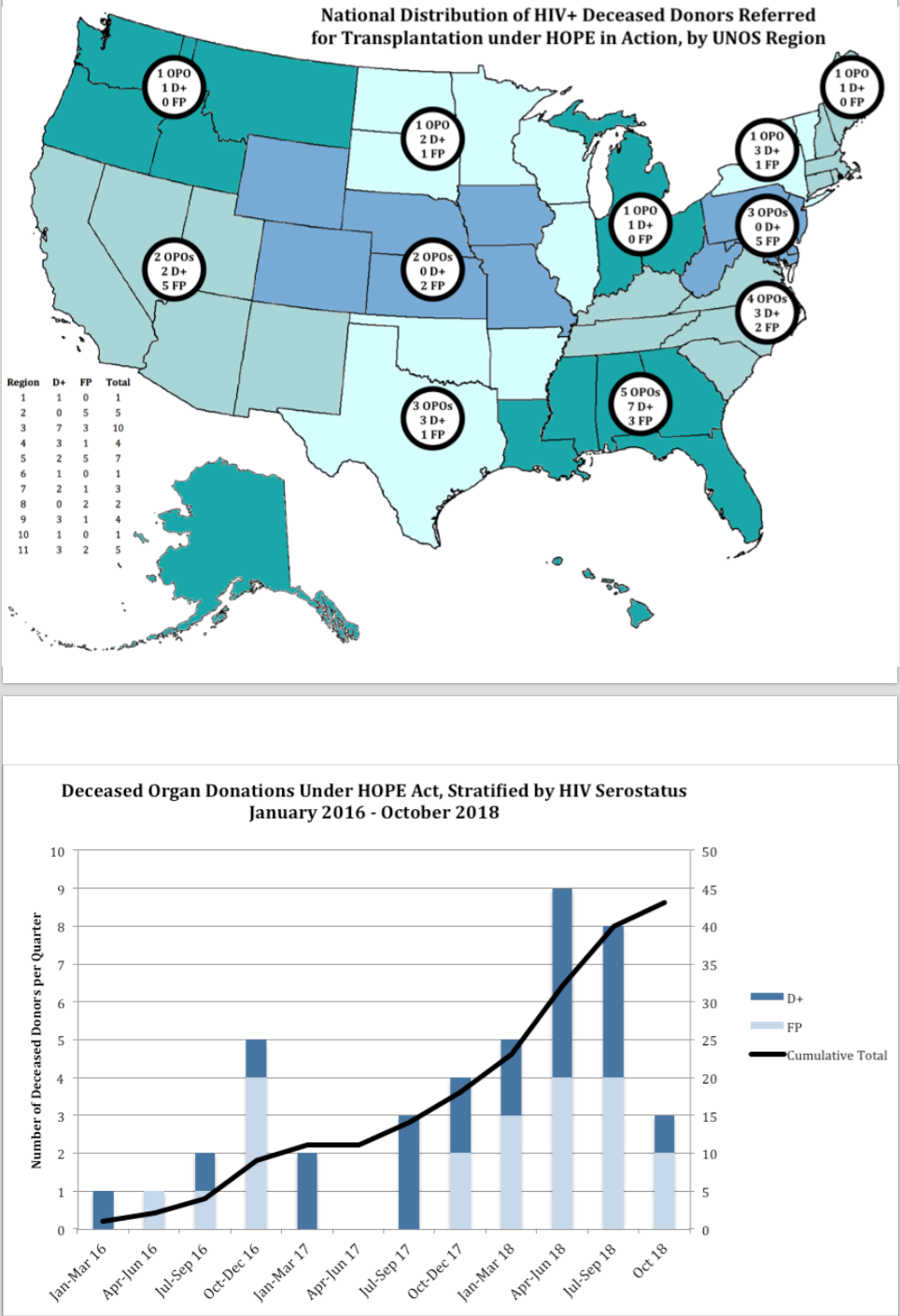
*Results: From 1/2016 to 10/2018, there were 83 donors: 23 D+, 20 FP and 40 D-, who had 129 organs recovered for transplant: 92 kidneys, 37 livers (Table 1). The number of annual HOPE Act donors increased from 9 in 2016 to 25 in 2018 (Figure 1). Overall median donor age was 33 (range 2-70). Median creatinine (1.0 mg/dL) and KDPI (40%) were similar across donor groups. Injection drug use (43% v 15 v 9) and HCV (25% v 5 v 4) were more common among D- donors (vs FP or D+, respectively). Among HIV D+, 17/23 (74%) were prescribed ART; 17% were discovered HIV diagnoses. Among HIV D+ with analyzed samples to date (N=14) median CD4 count was 218 cells/uL (IQR 124-362), CD4% 29.9 (IQR 23.9-36.1); 50% met AIDS criteria by absolute CD4 count, yet CD4% was discordantly high in many cases, with only 7% below the AIDS-defining CD4% threshold. 50% had HIV viral loads <40 copies/ml (9/18), including 8/11 donors on ART. 2/10 had X4 tropic virus. Major ART resistance mutations were detected in 6/19 patients, 1 with multiclass resistance (5%).
*Conclusions: Since initiation of HOPE Act trials in 2016, the number of HOPE donors has grown substantially. In contrast to the South African donor experience, nearly half are FP donors. Those with true infection are frequently ART experienced and resistance is common, yet very few donors had multiclass or integrase inhibitor resistance that could compromise standard recipient ART regimens.
To cite this abstract in AMA style:
Werbel WA, Kusemiju O, Barnaba B, Bowring M, Doby B, Kirby C, Fernandez R, Eby Y, Miller J, Brown D, Seaman S, Ostrander D, Tobian A, Segev D, Durand CM. Hope Act Donors With Organs Recovered For Transplant: The First Two Years [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/hope-act-donors-with-organs-recovered-for-transplant-the-first-two-years/. Accessed February 20, 2026.« Back to 2019 American Transplant Congress