Evaluation of Clinical and Safety Outcomes Following Uncontrolled Tacrolimus Conversion in Adult Transplant Recipients
A. Lichvar,1 S. Tremblay,2 D. Naik,2 E. King,4 A. Vinks,4 U. Christians,3 J. Lipscomb,4 R. Alloway.2
1U of Illinois at Chicago, Chicago, IL
2U of Cincinnati, Cincinnati, OH
3U of Colorado, Denver, CO
4Cincinnati Children's, Cincinnati, OH.
Meeting: 2018 American Transplant Congress
Abstract number: C291
Keywords: High-risk, Immunosuppression, Pharmacokinetics, Renal function
Session Information
Session Name: Poster Session C: Psychosocial and Treatment Adherence
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Long-term studies with control groups are required to assess tacrolimus (TAC) conversion. The purpose of this study was to compare clinical and safety outcomes of transplant (txp) recipients converted between different TAC products to those who were maintained on a single TAC formulation.
Methods: A retrospective, single-center study of txp recipients with pharmacy refill data was conducted from 08/09-05/16. Four groups were assessed: Group A) brand TAC, B) generic TAC (Sandoz), C) single conversion from brand name to generic, D) multiple conversions. Index date was either the date of first TAC product conversion (Groups C and D) or a pre-specified post-txp time (Groups A and B), selected by assessing the time to conversion post-txp in the intervention groups. Linear trend was estimated for dose-normalized TAC levels and eGFR using regression models allowing for differing slopes for each period (pre- vs post-index date) and across groups.
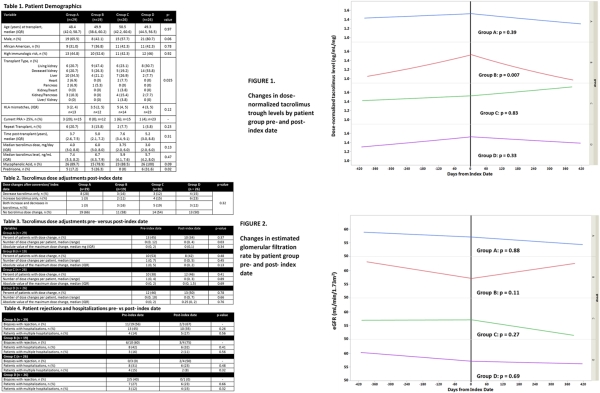
Results: 100 patients were included (A=29, B=19, C=26, D=26). Table 1 lists demographics. After the index date, 43% of patients had TAC dose modification and dose changes were similar across the groups (Table 2 and 3). No differences in hospitalization or rejection (Table 4). Linear trends in dose-normalized TAC levels across all 4 groups were similar (pre-index date period p=0.52; post-index date period p=0.08) (Figure 1) and in eGFR were similar (pre-index date p=0.65; post-index date period p=0.17) (Figure 2). P-values in Figures 1 and 2 represent pre- vs post index date by group. Group B had significantly lower troughs post-index date.
Conclusion: No significant differences were observed between any groups regarding dose modification, dose-normalized TAC trough concentrations, or other notable adverse drug effects. Significant dose-normalized TAC variability was observed in Group B pre-and post-index date periods, despite remaining on the same generic product.
CITATION INFORMATION: Lichvar A., Tremblay S., Naik D., King E., Vinks A., Christians U., Lipscomb J., Alloway R. Evaluation of Clinical and Safety Outcomes Following Uncontrolled Tacrolimus Conversion in Adult Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Lichvar A, Tremblay S, Naik D, King E, Vinks A, Christians U, Lipscomb J, Alloway R. Evaluation of Clinical and Safety Outcomes Following Uncontrolled Tacrolimus Conversion in Adult Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/evaluation-of-clinical-and-safety-outcomes-following-uncontrolled-tacrolimus-conversion-in-adult-transplant-recipients/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress