Urinary CXCL9 Monitoring to Accompany a Safe CNI-to-Belatacept Conversion in Kidney Transplant Patients
1Service de Néphrologie, Dialyse, Aphérèses et Transplantation, CHU Grenoble Alpes, Grenoble, France
2Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY.
Meeting: 2018 American Transplant Congress
Abstract number: C103
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Monitoring
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Converting renal transplant recipients from CNI to belatacept immunosuppression has been proposed as a strategy to limit nephrotoxicity, but it is associated with an increased risk of acute rejection. Urinary CXCL9 noninvasively detects early acute rejection and could be used to safely monitor patients during CNI-to-belatacept conversion.
Methods: In this prospective-cohort study, we enrolled EBV+ kidney transplant recipients with stable kidney function at >12 months after transplant and no DSA. Belatacept (5mg/kg) was administered day 0 (D0), D15, month 1 (M1) and then every four weeks, while CNI dose was halved on M1 then stopped at M2. We prospectively collected urine supernatants on D0, M1, M3, and M6 and quantified CXCL9 by ELISA.
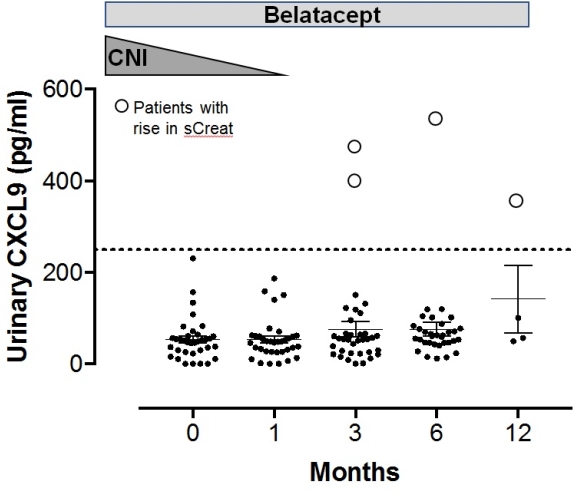
Results: From September 2016 to March 2017, we included 35 consecutive patients and all of them successfully switched from CNI to belatacept. 33 patients were on Tac, 2 on CsA. Mean eGFR was 45ml/min at D0, M3 46.8ml/min, and M6 45,6ml/min. Mean proteinuria was 0.3g/l at day0 and was unchanged at M6. The mean difference of eGFR between D0 and M6 was: -1,4ml/min; P=0.7. Two patients developed an acute rise in serum creatinine (sCreat >30%): one was interpreted as acute cellular rejection. In this patient, CXCL9 increased above 250pg/ml at 3 weeks before the rise in sCreat and lowered to normal levels at M6 following steroid pulses. The second patient had acute kidney dysfunction at month 4 which spontaneously resolved. CXCL9 was normal at D0 and M1, but flared at M3 and remained elevated. No biopsy was performed in this patient. All the other patients maintained stable kidney function and had normal CXCL9 levels (Figure 1).
Conclusions: Conversion from CNI to belatacept-based immunosuppression is feasible and safe, provided CNI weaning is performed slowly over a 2-month period. Monitoring urinary CXCL9 allows detection of early signs of immune-mediated graft dysfunction and may be used to guide conversion.
CITATION INFORMATION: Malvezzi P., Fischman C., Jouve T., Janbon B., Heeger P., Rostaing L., Cravedi P. Urinary CXCL9 Monitoring to Accompany a Safe CNI-to-Belatacept Conversion in Kidney Transplant Patients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Malvezzi P, Fischman C, Jouve T, Janbon B, Heeger P, Rostaing L, Cravedi P. Urinary CXCL9 Monitoring to Accompany a Safe CNI-to-Belatacept Conversion in Kidney Transplant Patients [abstract]. https://atcmeetingabstracts.com/abstract/urinary-cxcl9-monitoring-to-accompany-a-safe-cni-to-belatacept-conversion-in-kidney-transplant-patients/. Accessed March 6, 2026.« Back to 2018 American Transplant Congress