DSA Plus TCMR Leads to Poor Outcomes in Renal Allograft Recipients and This is Markedly Exacerbated by Non-Adherence
Starzl Transplant Institute, University of Pittsburgh, Pittsburgh.
Meeting: 2018 American Transplant Congress
Abstract number: C11
Keywords: Antibodies, Outcome, Rejection
Session Information
Session Name: Poster Session C: Histocompatibility and Immunogenetics
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Post-transplant DSA is strongly associated with poor renal allograft outcomes but has limited predictive value. In this prospective study we aimed to risk-stratify patients with early post-transplant DSA to allow timely identification of individuals at risk for poor clinical outcomes. Patients were screened for DSA at 0,1,3,6,9 &12mo and analyzed in relation to protocol biopsies at 3 and 12mos and any for-cause biopsies within the first year. IS was with Thymo and MPA+TAC.
67/294 (22.8%) of patients transplanted (01/2013-11/2014) developed DSA. Of these, 76% were detected within the first 3 months with 58% being persistent. DSA was associated with significantly increased rates of subclinical and clinical TCMR (58%) compared to patients lacking DSA (33%; p<0.0001). Importantly, presence of DSA was associated with higher Banff grade TCMR (Fig1A) and with higher rate of concomitant AMR (10.4% vs. 1.8%, p=0.003). In 77% of patients with both DSA and TCMR, DSA was detected at the same time or prior to TCMR. Patients with DSA and TCMR had significantly worse chronic allograft histological changes at 1 year (Fig1B) and significantly increased graft loss or impending graft loss (eGFR<30ml/min with >30% fall from baseline) at 4 yrs when compared to the others including patients with TCMR or DSA alone (Fig 1C). In a multivariate Cox model, younger recipient age, Class I HLA mismatch, DGF and poor adherence (defined by a high intra-patient CNI variability (>35%)) were independently associated with DSA plus TCMR. Of these, non-adherence, which is potentially modifiable, further risk stratifies patients with DSA plus TCMR. In fact, >70% of nonadherent patients with DSA plus TCMR have impending/graft loss by 4 yrs, whereas patients with DSA plus TCMR who are adherent have outcomes comparable to other patient groups (Fig1D). Thus, early post-transplant DSA is associated with increased TCMR which in turn leads to poor graft outcomes, particularly in nonadherent patients. Strategies to address non-adherence in patients with DSA plus TCMR should be explored to improve long-term graft outcomes. 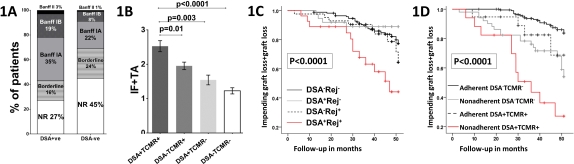
CITATION INFORMATION: Chittka D., Cherukuri A., Sharma A., Mehta R., Hariharan S., Rothstein D. DSA Plus TCMR Leads to Poor Outcomes in Renal Allograft Recipients and This is Markedly Exacerbated by Non-Adherence Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Chittka D, Cherukuri A, Sharma A, Mehta R, Hariharan S, Rothstein D. DSA Plus TCMR Leads to Poor Outcomes in Renal Allograft Recipients and This is Markedly Exacerbated by Non-Adherence [abstract]. https://atcmeetingabstracts.com/abstract/dsa-plus-tcmr-leads-to-poor-outcomes-in-renal-allograft-recipients-and-this-is-markedly-exacerbated-by-non-adherence/. Accessed February 1, 2026.« Back to 2018 American Transplant Congress
