Use of Hepatitis-C Treatments after Kidney Transplantation: Impact of the Direct-Acting Antiviral Era
K. Lentine, D. Axelrod,2 R. Bloom,3 H. Xiao,1 D. Segev,4 T. Alhamad,5 H. Randall,1 R. Ouseph,1 V. Dharnidharka,5 D. Brennan,4 N. Lam,6 A. Naik,7 G. Hess,8 B. Kaiske,9 M. Schnitzler.1
1Saint Louis U, St. Louis
2Lahey, Burlington
3U Penn, Philadelphia
4Johns Hopkins, Baltimore
5Washington U, St. Louis
6U Alberta, Edmonton, Canada
7U Michigan, Ann Arbor
8Hennepin, Minneapolis
9Symphony Health, Conshohocken.
Meeting: 2018 American Transplant Congress
Abstract number: 42
Keywords: Donors, Hepatitis C, Kidney transplantation, marginal, Resource utilization
Session Information
Session Name: Concurrent Session: Kidney Infectious - Viral Hepatitis
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:54pm-3:06pm
Presentation Time: 2:54pm-3:06pm
Location: Room 4C-4
Hepatitis C virus (HCV) infection in kidney transplant (KTx) donors or recipients has correlated with adverse outcomes, partly due to limited treatment options. Development of direct-acting antiviral agents (DAAs) revolutionized management of HCV, offering safe and effective therapies that may be used after KTx.
We integrated SRTR data with records from a national pharmaceutical claims warehouse (2008-2016) to identify HCV treatments after KTx. Pharmacy fills for of HCV treatments were assessed before and after 1/2014, representing pre-DAA (N=100,497) and post-DAA (N=57,367) eras. Treatment patterns were stratified by donor (D) and recipient (R) HCV serostatus.
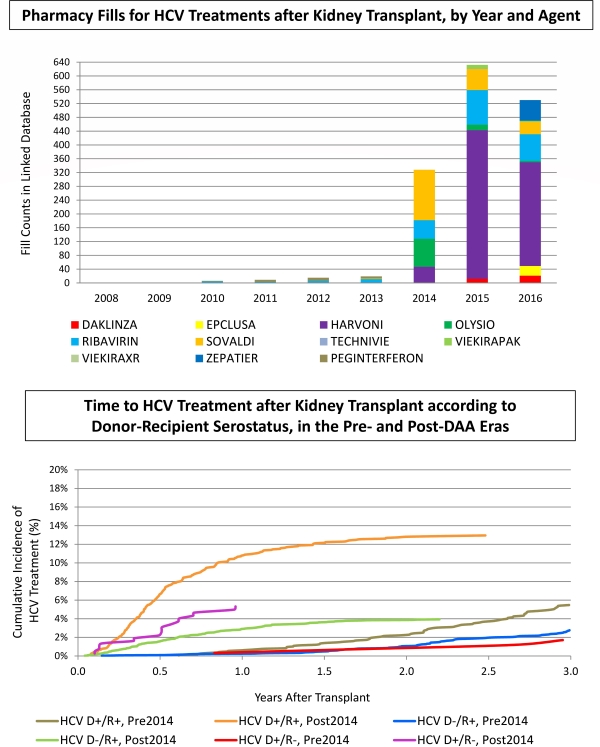
Pharmacy fills for HCV treatments after KTx increased sharply in the post-DAA era, driven by sofosbuvir regimens, along with newer agents approved in 2016 (Fig 1).
Among HCV D+/R+ recipients, the cumulative incidence of HCV treatment by 1yr post-KTx increased >10-fold in the post-DAA era, from 0.6% to 10.8% (Fig 2). HCV treatment also increased 10-fold in HCV D-/R+ recipients, from 0.3% to 3.0% at 1yr post-KTx. HCV D+/R- KTx was uncommon (0.2% and 0.5% of KTx in the pre- and post-DAA eras, respectively), but HCV treatments also increased in this group in the post-DAA era (from 0.3% to 5.3% by 1yr). In parallel with expanded HCV treatment options, HCV D+ KTx rose from 1.6% to 2.4% after 1/2014.
Among treated patients, average spending for HCV therapy over the 1st year post-KTx was markedly higher in the post-DAA era ($103,495 vs $5,411).
Development of DAAs is associated with increased post-KTx HCV treatment, D+ KTx, and treatment-related costs. Future work should explore impact of HCV management and organ use strategies on waitlist and post-KTx survival, and cost-effectiveness.
CITATION INFORMATION: Lentine K., Axelrod D., Bloom R., Xiao H., Segev D., Alhamad T., Randall H., Ouseph R., Dharnidharka V., Brennan D., Lam N., Naik A., Hess G., Kaiske B., Schnitzler M. Use of Hepatitis-C Treatments after Kidney Transplantation: Impact of the Direct-Acting Antiviral Era Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Lentine K, Axelrod D, Bloom R, Xiao H, Segev D, Alhamad T, Randall H, Ouseph R, Dharnidharka V, Brennan D, Lam N, Naik A, Hess G, Kaiske B, Schnitzler M. Use of Hepatitis-C Treatments after Kidney Transplantation: Impact of the Direct-Acting Antiviral Era [abstract]. https://atcmeetingabstracts.com/abstract/use-of-hepatitis-c-treatments-after-kidney-transplantation-impact-of-the-direct-acting-antiviral-era/. Accessed February 12, 2026.« Back to 2018 American Transplant Congress