Investigation of Lower Treatment Failure Rate Seen among Black Kidney Recipients on LCP-Tacrolimus vs. IR-Tacrolimus: Subgroup Analyses of Two Phase 3 Trials
1University of Maryland, Baltimore, MD
2Veloxis, Cary, NC
3Medstar Georgetown Transplant Institute, Washington, DC.
Meeting: 2018 American Transplant Congress
Abstract number: 39
Keywords: African-American, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: mTORi Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 6A
Black kidney transplant recipients are recognized as high risk for inferior allograft survival due to immunologic and non-immunologic factors. Pooled data from 2 Phase 3 studies showed fewer treatment failures in black recipients on the once-daily tacrolimus formulation, LCPT, compared to those on IR-TAC (4.5% vs. 18.4%, p=0.054). This study evaluated tacrolimus exposure and outcomes in the black recipients from these studies in order to investigate this difference in treatment failure.
Tacrolimus levels, efficacy and safety outcomes were obtained from Phase 3 trials evaluating LCPT vs. IR-TAC in stable and de novo kidney recipients. A conversion multiplier of 0.85 was used in stable patients and initial total daily dosages of 0.17 mg/kg/day and 0.1 mg/kg/day of LCPT and IR-TAC, respectively, were used in the de novo study.
93 recipients were included. LCPT and IR-TAC groups were similar with respect to age, gender, donor type, and sensitization. Biopsy-proven acute rejections (BPAR) largely accounted for the difference in efficacy, accounting for treatment failures in 3 stable IR-TAC patients, 3 de novo IR-TAC patients, and only 1 de novo LCPT patient. No differences in mean tacrolimus troughs between stable patients on LCPT or IR-TAC were seen over the study period. In contrast, de novo LCPT patients had higher levels during the first 2 post-transplant weeks. Despite more rapid therapeutic levels achieved for LCPT, DGF occurred in none of the LCPT patients and 4 IR-TAC patients; renal function was similar throughout the first post-transplant year. Treatment-emergent AE rates were similar between LCPT (91%) and IR-TAC groups (98%). Common (>20%) AEs included diarrhea for IR-TAC (24.5%), and constipation (20.5%) and headache (20.5%) for LCPT patients.
Reduced treatment failures in black recipients on LCPT are primarily due to reduction in BPAR in both de novo and maintenance settings. These data may help elucidate the efficacy difference seen between black recipients on LCPT versus those on IR-TAC. 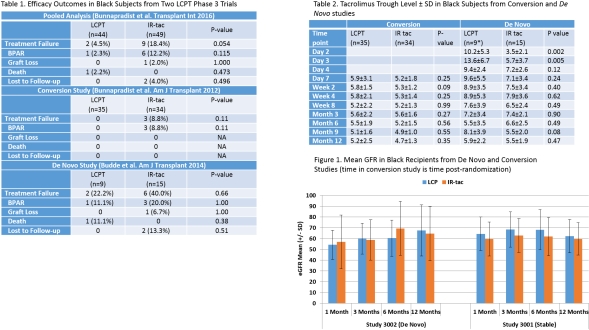
CITATION INFORMATION: Sparkes T., Masters B., Staino C., Patel S., Cooper M. Investigation of Lower Treatment Failure Rate Seen among Black Kidney Recipients on LCP-Tacrolimus vs. IR-Tacrolimus: Subgroup Analyses of Two Phase 3 Trials Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sparkes T, Masters B, Staino C, Patel S, Cooper M. Investigation of Lower Treatment Failure Rate Seen among Black Kidney Recipients on LCP-Tacrolimus vs. IR-Tacrolimus: Subgroup Analyses of Two Phase 3 Trials [abstract]. https://atcmeetingabstracts.com/abstract/investigation-of-lower-treatment-failure-rate-seen-among-black-kidney-recipients-on-lcp-tacrolimus-vs-ir-tacrolimus-subgroup-analyses-of-two-phase-3-trials/. Accessed February 11, 2026.« Back to 2018 American Transplant Congress
