A Novel, Dose-Adjusted Tacrolimus Trough Concentration (TTC) Model for Prediction and Variance Estimation.
1Astellas Pharma Global Development, Inc., Northbrook, IL
2Astellas Pharma Global Development, Inc., Chertsey, United Kingdom
Meeting: 2017 American Transplant Congress
Abstract number: D91
Keywords: Immunosuppression, Kidney transplantation, Monitoring, Prediction models
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Given that a high tacrolimus (tac) intrapatient variability (IPV) increases the risk for a poor kidney transplant (tx) outcome, some experts advocate routine IPV monitoring for detection of high-risk patients. However, attempts to predict TTC variance (var) are limited by the need for patients to receive a fixed dose over time, or use conventional, linear statistical models.
This study analyzes dose-adjusted TTC using a novel nonparametric functional regression model with TTC as a response and dose over time as a covariate. The model represents this relationship using an unspecified bivariate function, from which we derive a dose-adjusted var of TTC based on standard functional principal component analysis (FPCA). To assess the model, it was compared against a (+)-control (FPCA-only) and standard linear, mixed effect models (LMEs) using data from Astellas NDA studies for Astagraf XL® (prolonged-release tacrolimus).
The new model showed improved prediction accuracy over LMEs. When var was high, as in the first 10 days post-tx, LMEs typically performed poorly. However, the flexibility of the new model accounted for the var of TTC during this period of large dose fluctuations. This was illustrated by estimated coverage probabilities from the new model that were close to the true nominal levels unlike the underestimated coverage probabilities of the LMEs. Figure (a) illustrates the full model and Figure (b) illustrates the subject-level bias of an LME that over-predicts TTC by 0.5–2ng/mL versus the new model predicting within 0–1ng/mL.
This is the first known use of functional regression modeling to estimate dose-adjusted TTC and its var. Unlike all known conventional methodologies, our model accounts for a possible complex, nonlinear relationship between TTC and dose, and this approach is generalizable to real-world situations due to its minimal modeling assumptions. Future extensions of this research aim to characterize the effect of tac IPV on tx outcomes, and improve understanding of factors that impact dose-adjusted TTCs at the individual subject level.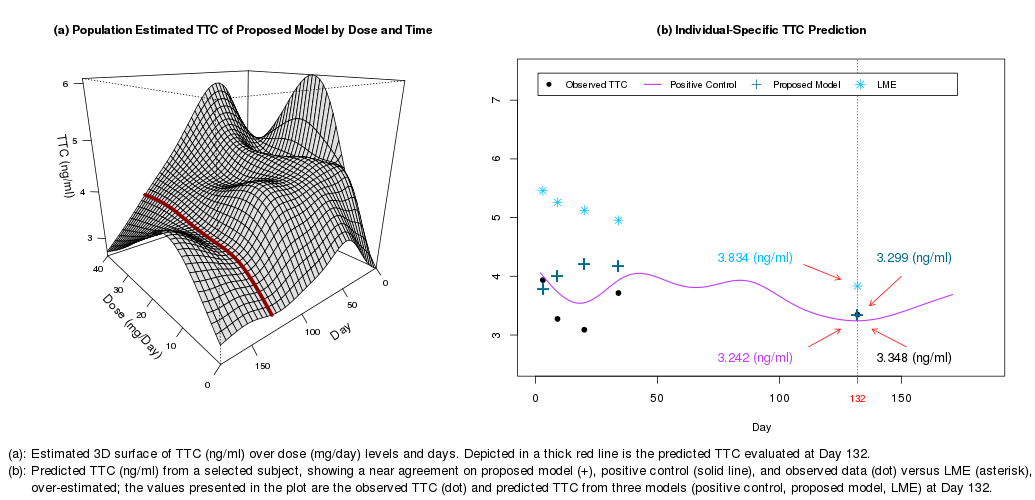
CITATION INFORMATION: Kim J, Wilson S, Undre N, Fei S, Kristy R, Schwartz J. A Novel, Dose-Adjusted Tacrolimus Trough Concentration (TTC) Model for Prediction and Variance Estimation. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kim J, Wilson S, Undre N, Fei S, Kristy R, Schwartz J. A Novel, Dose-Adjusted Tacrolimus Trough Concentration (TTC) Model for Prediction and Variance Estimation. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-novel-dose-adjusted-tacrolimus-trough-concentration-ttc-model-for-prediction-and-variance-estimation/. Accessed February 8, 2026.« Back to 2017 American Transplant Congress
