Kidney Transplantation in HCV+ Recipients in a New Era of Diagnostics and Therapeutics.
C. Traynor, S. Bumb, C. Berg, M. Ellis, J. Roberts, A. Muir, L. King, C. Brady, S. Sanoff.
Duke University Medical Center, Durham, NC
Meeting: 2017 American Transplant Congress
Abstract number: A296
Keywords: Hepatitis C, Kidney transplantation
Session Information
Session Name: Poster Session A: Viral Conundrums
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
Background
Hepatitis-C infected persons (HCV+) evaluated for kidney transplantation receive rigorous staging of their liver disease, often including liver biopsy. Meanwhile, studies have shown that despite careful selection, patients receiving organs from HCV+ donors historically had worse patient/graft survival. This lead to underutilization of HCV+ donors, which offer shorter waiting times.
Ultrasound technology permits less invasive testing and new Direct Acting Antiviral Agents (DAAs) give the potential to effectively treat HCV post transplant. Using these innovations, our transplant program began offering streamlined evaluation and access to HCV+ organs for HCV+ candidates, and timely treatment with DAAs. The aim of our study is to review the evaluation and outcome of HCV+ recipients in this new era.
Methods
This is a retrospective study of adult HCV+ kidney transplant recipients (deceased donor) at our institution between January 1, 2014 and June 11, 2016, allowing for 6-months of follow-up. Data includes fibroscan/liver biopsy results in the evaluation phase, donor characteristics and clinical outcomes (creatinine, proteinuria, sustained virologic response).
Results
During the study period, 25 HCV+ patients were transplanted-baseline demographics, transplant data, and outcomes are outlined in Table 1. Of 8 patients who had a fibroscan, 4 avoided liver biopsy. In the cohort, 17 received HCV+ organs. Median wait time was 466 vs. 2169 days (HCV+ vs. HCV- donor). Rates of delayed graft function were higher in with HCV+ organs, with similar rejection rates. There was 100% graft/patient survival at 6-months and 1-year.
Conclusion
In the modern era of HCV diagnostics and therapeutics, many HCV+ kidney transplant candidates may be evaluated without invasive liver biopsies. By delaying treatment with DAA until after transplant, HCV+ recipients may reduce their time to transplant by accepting an HCV+ organ, with excellent short-term patient and graft survival. Longer follow-up is required to see if treatment with DAAs after transplant produces similar long-term outcomes between recipients of HCV+ and HCV- donors.
(Note: 1st and 2nd authors contributed equally)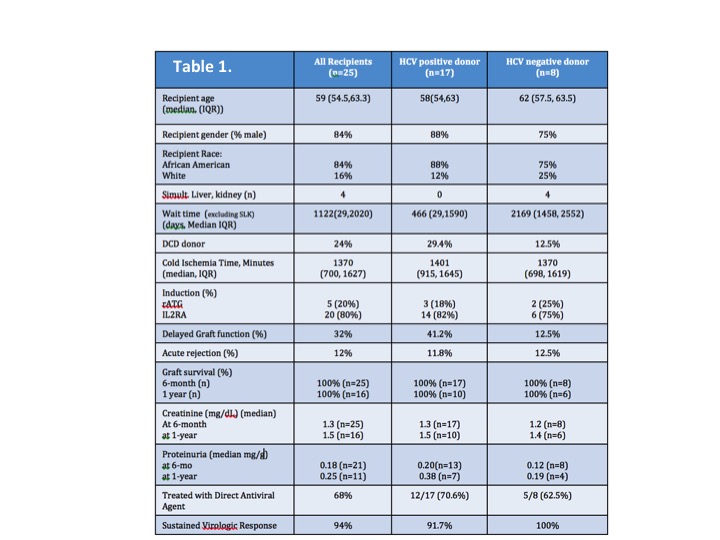
CITATION INFORMATION: Traynor C, Bumb S, Berg C, Ellis M, Roberts J, Muir A, King L, Brady C, Sanoff S. Kidney Transplantation in HCV+ Recipients in a New Era of Diagnostics and Therapeutics. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Traynor C, Bumb S, Berg C, Ellis M, Roberts J, Muir A, King L, Brady C, Sanoff S. Kidney Transplantation in HCV+ Recipients in a New Era of Diagnostics and Therapeutics. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/kidney-transplantation-in-hcv-recipients-in-a-new-era-of-diagnostics-and-therapeutics/. Accessed February 28, 2026.« Back to 2017 American Transplant Congress
