Mycophenolate Dose Reductions Are Not Associated with De Novo Donor-Specific Antibodies.
University of Colorado, Aurora, CO
Meeting: 2017 American Transplant Congress
Abstract number: A26
Keywords: Antibodies, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Antibody Mediated Rejection in Kidney Transplant Recipients I
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
De novo donor-specific antibodies (dnDSA) are central to the development of antibody-mediated rejection, which is the most important cause of death-censored graft loss. It is common practice to reduce the standard dose of mycophenolate due to temporary complications, yet it is unknown whether this practice places patients at increased risk for dnDSA. The purpose of this study was to determine if mycophenolate dose reduction was associated with dnDSA and other adverse outcomes.
From 2007 to 2013, 550 kidney transplant recipients initiated on mycophenolate sodium (720 mg twice daily) or mycophenolate mofetil (1000 mg twice daily) in addition to a calcineurin inhibitor (usually tacrolimus) and prednisone were prospectively screened for dnDSA at months 1, 6, 12, yearly, and when clinically indicated. Dose reductions were categorized as a percentage of the standard dose that it was reduced (25%, 50%, 75%, or held). dnDSA was considered positive if it occurred after a dose reduction. Logistic regression was used to calculate odds ratios for dnDSA and time dependent covariate Cox proportional hazards regression models were used for acute rejection and death-censored graft survival analysis, which were adjusted for age, gender, ethnicity, donor type, induction therapy, and delayed graft function.
212 patients (38.5%) patients had a reduction in their mycophenolate dose, with 35 (6.4%) patients with 25% reduction of standard dose, 169 (30.7%) patients with 50%, and 8 (1.5%) patients with 75%. Any dose reduction (OR 0.94, 95% CI 0.62-1.44, p=0.786), 25% (OR 1.49 95% CI 0.61-3.68, p=0.382), 50% (OR 0.86, 95% CI 0.55-1.35, p=0.871), and 75% (OR 1.23, 95% CI 0.15-9.87, p=0.849) reduction of standard dose was not associated with increased risk of dnDSA. Holding mycophenolate (OR 0.80, 95% CI 0.34-1.91, p=0.622) did not have an association with dnDSA but was associated with acute rejection (HR 3.84, 95% CI 1.83-8.03, p<0.001) and death-censored graft loss (HR 5.1, 95% CI 2.30-11.30, p<0.001).
These results suggest that reducing the standard dose of mycophenolate is not associated with an excess risk of dnDSA but may increase the risk of acute rejection and graft loss.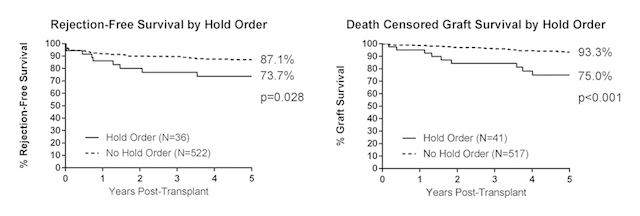
CITATION INFORMATION: Davis S, Gralla J, Klem P, Wiseman A, Cooper J. Mycophenolate Dose Reductions Are Not Associated with De Novo Donor-Specific Antibodies. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Davis S, Gralla J, Klem P, Wiseman A, Cooper J. Mycophenolate Dose Reductions Are Not Associated with De Novo Donor-Specific Antibodies. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/mycophenolate-dose-reductions-are-not-associated-with-de-novo-donor-specific-antibodies/. Accessed February 24, 2026.« Back to 2017 American Transplant Congress
