A Novel Belatacept Regimen to Optimize Efficacy and Safety.
1MGH, Boston
2UCSF, San Francisco
Meeting: 2017 American Transplant Congress
Abstract number: 540
Keywords: Kidney transplantation
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Belatacept
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: E354b
Despite superior long term graft survival when compared to cyclosporine, the use of the FDA approved belatacept regimen in clinical practice has been constrained by higher rates of acute rejection. We hypothesized that the use of rATG and an mTORi with belatacept would improve efficacy and provide comparable safety.
Methods
Retrospective single center analysis of the first 44 EBV seropositive, low immunologic risk kidney transplant recipients treated with belatacept combined with rATG induction (3 mg/Kg) and initial MMF maintenance with conversion to everolimus at 1 month post-transplant ± corticosteroids. Biopsies were performed for cause. GFR was estimated by the MDRD equation. A subset of patients who agreed to immune monitoring were classified by 2 different gene expression assays: (1) the kidney solid organ response test/kSORT (peripheral blood) and (2) urine common rejection module/uCRM as being positive (kSORT ≥9, uCRM ≥4; high risk for rejection) or negative (immune quiescence).
Results
The cohort was 59% male, 43% Caucasian, with a mean age of 57 years and low immunologic risk (mean cPRA 6%). Diabetes was the most common cause of ESRD (39%) followed by PKD (23%). 54% received a deceased donor kidney transplant; 28% developed DGF. The mean 1 year eGFR was 61.4 (SD 18.4) mL/min/1.73 m2. 12 cause biopsies were performed with 5 acute cellular rejections (11.4%).
| Cause Biopsy, n | 12 |
| No rejection, n | 2 |
| Borderline, n | 4 |
| ACR, n | 5 |
| 1a | 2 |
| 1b | 1 |
| 2b | 2 |
| BKVAN, n | 1 |
All rejections occurred in patients still on MMF or changed from everolimus; 2 rejections occurred in patients off steroids. There was 1 patient death. 32% developed BK viremia and 12% developed CMV viremia. No fungal infections were seen. There were no cases of PTLD. 27 patients underwent kSORT and uCRM testing, with samples obtained at a mean of 731 (range 169-1335) days post-transplant.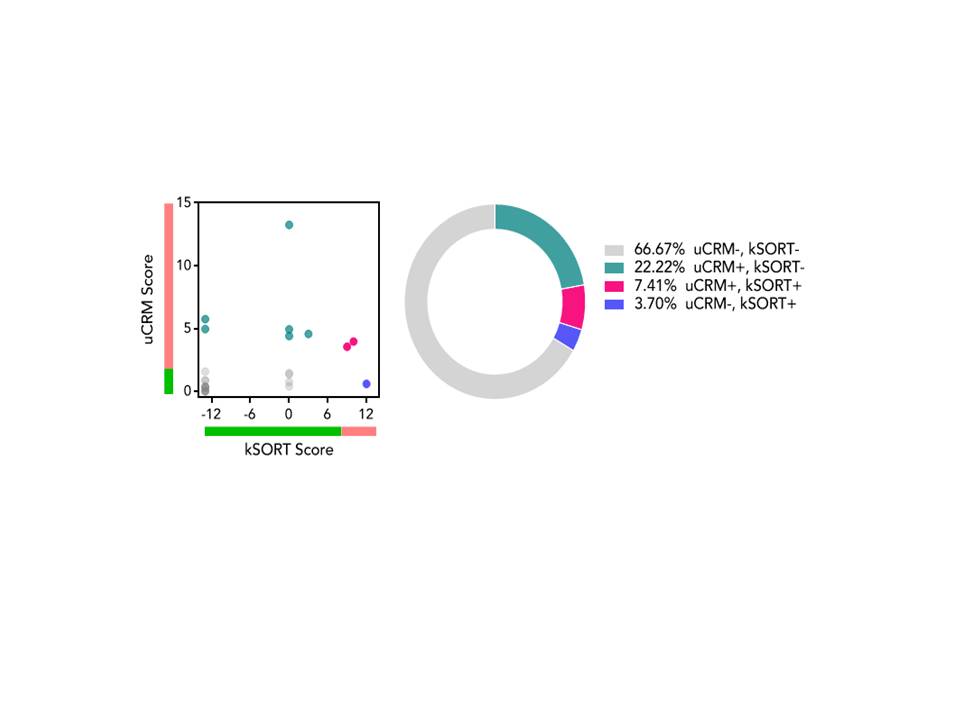 Conclusion
Conclusion
A novel belatacept regimen with low dose rATG induction and maintenance everolimus demonstrated a low acute rejection rate and maintained an excellent 1-year eGFR with evidence of immune quiescence in a majority of patients.
CITATION INFORMATION: Wojciechowski D, Chandran S, Sarwal M, Yang J, Vincenti F. A Novel Belatacept Regimen to Optimize Efficacy and Safety. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Wojciechowski D, Chandran S, Sarwal M, Yang J, Vincenti F. A Novel Belatacept Regimen to Optimize Efficacy and Safety. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-novel-belatacept-regimen-to-optimize-efficacy-and-safety/. Accessed February 27, 2026.« Back to 2017 American Transplant Congress
