Belatacept Conversion for Calcineurin Inhibitor (CNI) Toxicity: Our First 100 Patients.
Columbia University, New York
Meeting: 2017 American Transplant Congress
Abstract number: 538
Keywords: Immunosuppression, Kidney transplantation, Renal function, Toxocity
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Belatacept
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: E354b
Intro: CNIs improve kidney transplant (KT) survival, but have significant toxicities, including graft dysfunction (GD), neurotoxicity (NT), or thrombotic microangiopathy (TMA). Belatacept is an alternative that has been used to minimize CNI exposure. We reviewed our experience with belatacept conversion (BC) in 100 patients (pts).
Methods/Results: Since 2013, 100 pts on CNI based regimens underwent BC a median 8.5 [3.8-27] mo after KT: 77 for GD, 10 for TMA, 13 for NT. Patients were 52.4 [37.7-61.5] yrs old, 57 were male, 64 received DDKT, induction was primarily either alemtuzumab (52) or thymoglobulin (41). Pts were followed for a median of 8.9 [5.5-21] mo after BC. 45 remained on lower dose CNI, 60 were also on prednisone, and 93 were on an antimetabolite.
Creatinine (Cr) improved significantly from 2.9 to 2.0 mg/dl (p < 0.0001) by 30 days and to 1.7 (p<0.0001) at last f/u. 81 patients had improvement in Cr and 31 pts had >30% improvement in Cr at last follow-up. 89 pts had biopsies prior to BC. On biopsy, >25% glomerulosclerosis predicted lack of 30% improvement (p = 0.046), and >25% IFTA or vascular disease predicted allograft failure (p = 0.002). No other biopsy findings were associated with outcomes. Proteinuria did not change after BC (0.91 vs 0.82, p=0.47) nor did it predict response to conversion.
After BC, 18 pts had rejection (all ACR), at median 4.5 mo [2.1 – 9.2]. 11 of these 18 had a prior rejection, 9 within 3 mo of conversion. 7 pts had allograft failure 6.4 [5.2 – 9.7] mo after BC, including 1 cardiac death (MI). 5 pts developed BK viremia 9.7 [7.8 – 10.1] mo after BC, and 10 pts developed CMV viremia 5.2 [3.3 – 12] mo after BC. Presence of DSA prior to BC did not predict allograft rejection post BC. Higher immunologic risk pts requiring maintenance prednisone had higher rejection rates (p=0.045). Continuation of lower dose CNI or antimetabolite was not associated with rejection.
Conclusion: Overall, there was improvement in Cr for pts after BC, with 31 demonstrating >30% improvement. Further analysis into the cause of KT rejection and failure in these pts may aid in informing a strategy to convert pts to belatacept after KT complicated by toxicity or rejection.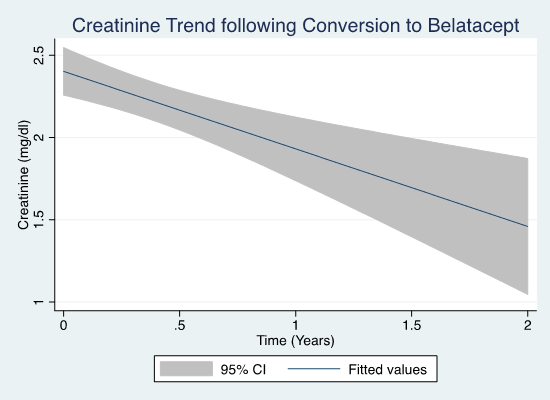
CITATION INFORMATION: Crew R, Patel S, Pai A, Batal I, Fernandez H. Belatacept Conversion for Calcineurin Inhibitor (CNI) Toxicity: Our First 100 Patients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Crew R, Patel S, Pai A, Batal I, Fernandez H. Belatacept Conversion for Calcineurin Inhibitor (CNI) Toxicity: Our First 100 Patients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-conversion-for-calcineurin-inhibitor-cni-toxicity-our-first-100-patients/. Accessed February 24, 2026.« Back to 2017 American Transplant Congress
