Experience with Belatacept (CTLA4-Ig) Conversion in Highly HLA Sensitized (HS) and ABO-Incompatible (ABOi) Patients Who Received Incompatible Kidney Transplants Post Desensitization (DES).
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2HLA Laboratory, Cedars-Sinai Medical Center, Los Angeles, CA
Meeting: 2017 American Transplant Congress
Abstract number: 537
Keywords: Kidney transplantation
Session Information
Session Name: Concurrent Session: Novel Immunosuppression Regimens - Belatacept
Session Type: Concurrent Session
Date: Tuesday, May 2, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: E354b
Introduction: Recent experience with belatacept (bela) in nonsensitized kidney tx recipients suggest that costimulatory blockade in a CNI-free environment results in superior graft survival & improved renal function at 7 years compared to standard CNI (Vicenti et al NEJM 2016; 375(4):333-43). In addition significant reductions in de novo DSAs were seen in the bela treatment group. However there are no reports of bela use in HS & ABOi pts. after DES. Here we report our experience with bela conversion to CNI-free regimen in HS & ABOi pts. Methods: From 2012 to present we converted 62 pts to a CNI-free regimen using bela. Briefly pts. meeting criteria for conversion:CNI toxicity & aHUS received bela 5mg/kg monthly with CNI reduced to 50% at first & discontinued at 2nd bela dose. Pts were monitored for renal function, viral pcrs, & DSAs. We identified 11 HS and 2 ABOi patients. Results: A reduction in serum Cr values at baseline to 3 and 6 months post conversion which was not statistically significant. DSA levels in HS patients pre & post bela conversion. All DSAs showed reductions compared to baseline levels. DSAs are shown in Figure 1. Only 1 patient who had an initial biopsy showing ABMR at 5Y post tx with declining renal function showed progression of ABMR with TG. One pt received ABOi & HLAi kidney tx from a living donor & was initiated with bela at transplantation due aHUS & initial DSAs have disappeared & anti-A titers significantly declined at 6M post tx. No evidence of rejection & renal dysfunction has been seen. Conclusions: Conversion to CNI-free regimen with bela in HS & ABOi kidney transplant patients is successful without evidence of initiation of ongoing rejections. Reductions in DSA levels post bela is encouraging suggesting a possible inhibitory mechanism of bela on antibody producing cells. Finally, further studies of bela therapy in HS & ABOi pts is warranted.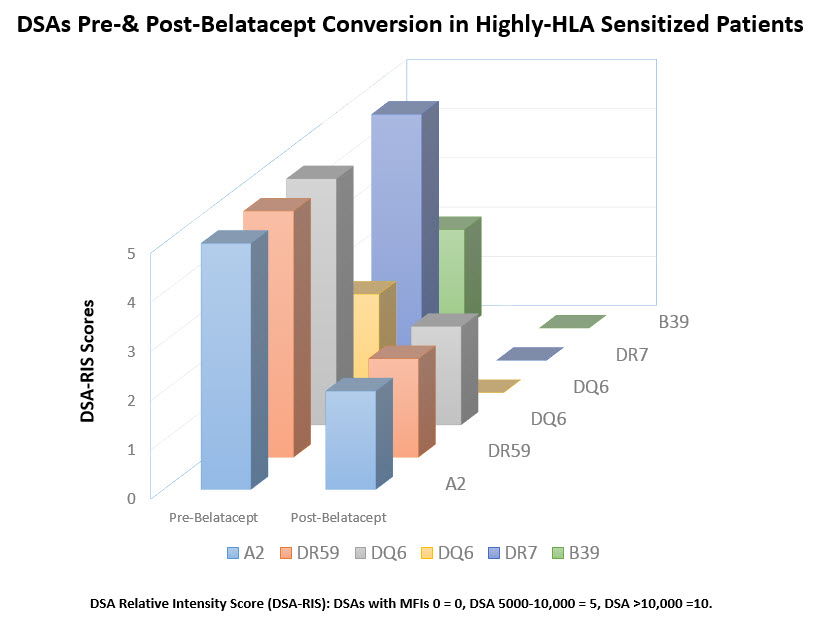
CITATION INFORMATION: Choi J, Peng A, Vo A, Huang E, Najjar R, Louie S, Kang A, Ge S, Toyoda M, Zhang X, Jordan S. Experience with Belatacept (CTLA4-Ig) Conversion in Highly HLA Sensitized (HS) and ABO-Incompatible (ABOi) Patients Who Received Incompatible Kidney Transplants Post Desensitization (DES). Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Choi J, Peng A, Vo A, Huang E, Najjar R, Louie S, Kang A, Ge S, Toyoda M, Zhang X, Jordan S. Experience with Belatacept (CTLA4-Ig) Conversion in Highly HLA Sensitized (HS) and ABO-Incompatible (ABOi) Patients Who Received Incompatible Kidney Transplants Post Desensitization (DES). [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/experience-with-belatacept-ctla4-ig-conversion-in-highly-hla-sensitized-hs-and-abo-incompatible-aboi-patients-who-received-incompatible-kidney-transplants-post-desensitization-des/. Accessed January 21, 2026.« Back to 2017 American Transplant Congress
