Multiple Dosing of Anti-C1s Antibody TNT009 – Effect on HLA Antibody-Triggered Complement Activation in Healthy Volunteers and Kidney Transplant Recipients with Antibody-Mediated Rejection.
1Department of Surgery, Medical University Vienna, Vienna, Austria
2Department of Clinical Pharmacology, Medical University Vienna, Vienna, Austria
3Department of Medicine III, Medical University Vienna, Vienna, Austria
4True North Therapeutics, Inc., San Francisco, CA
Meeting: 2017 American Transplant Congress
Abstract number: 168
Keywords: Alloantibodies, Humanized antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Novel Immunosuppression - DSA Monitoring
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: E450b
Study purpose. Complement inhibition may be an attractive strategy to prevent antibody-mediated allograft injury. One promising therapeutic target may be the enzymatic activity of key classical pathway (CP) component C1. In this first-in-human trial (NCT02502903) we evaluated the impact of 4 weeks treatment with TNT009, a humanized anti-C1s antibody on CP activity in healthy individuals and kidney transplant recipients with late antibody-mediated rejection (ABMR). Methods. Sixteen healthy volunteers received 4 weekly IV doses of TNT009 or placebo (6:2 randomization, two cohorts: 30 or 60 mg/kg). Subsequently, 10 kidney transplant recipients diagnosed with late ABMR were treated with TNT009 (initial test dose of 10 mg/kg followed by 4 weekly doses of 60 mg/kg). To assess ex vivo HLA antibody-triggered complement activation, sera from dosed subjects were analysed on microbeads sensitized with HLA antibodies using the read-out of C3d fixation. Results. Baseline C3d deposition levels were not significantly different between healthy volunteers and transplant recipients (mean C3d MFI: 4882±754 versus 4367±1030, p=0.15). In healthy volunteers, multiple doses of TNT009 led to a persistent (≥4 weeks) and >80% inhibition of HLA antibody-triggered C3d deposition (and in parallel CH50 activity). Similarly, sustained complement inhibition was also achieved in the cohort of ABMR patients, with a comparable degree of CP blockade. 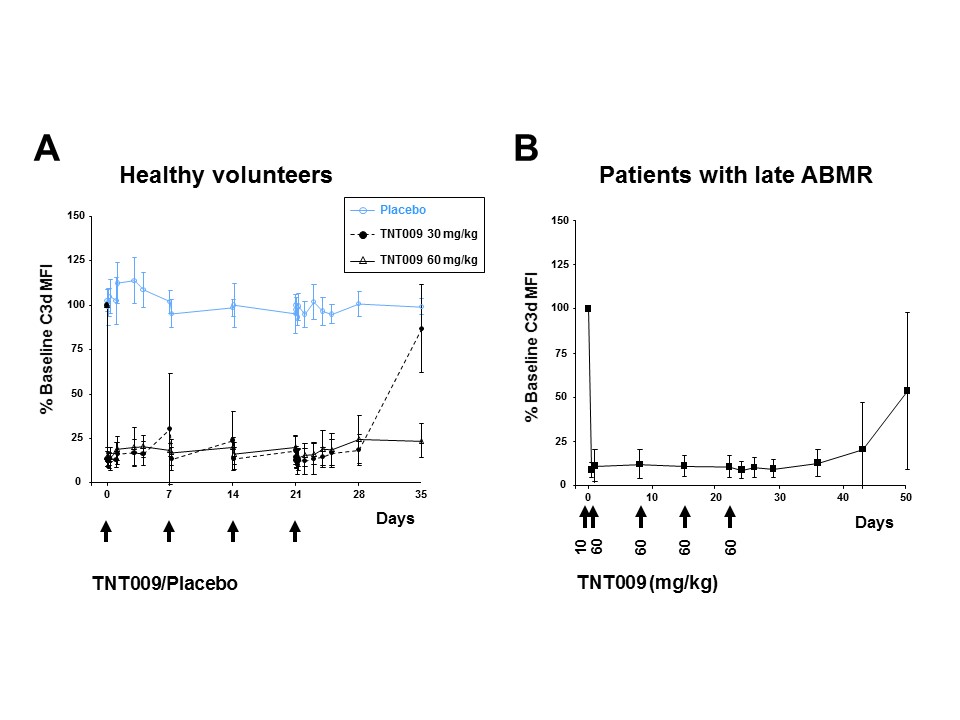 CP inhibition tightly correlated with plasma levels of TNT009. Conclusion. Multiple doses of TNT009 allowed for a prolonged and near complete CP inhibition both in healthy volunteers and ABMR patients. Our results provide a valuable basis for future studies evaluating the effect of prolonged C1s blockade on the course of antibody-mediated rejection.
CP inhibition tightly correlated with plasma levels of TNT009. Conclusion. Multiple doses of TNT009 allowed for a prolonged and near complete CP inhibition both in healthy volunteers and ABMR patients. Our results provide a valuable basis for future studies evaluating the effect of prolonged C1s blockade on the course of antibody-mediated rejection.
CITATION INFORMATION: Muhlbacher J, Jilma B, Wahrmann M, Eskandary F, Gilbert J, Panicker S, Böhmig G. Multiple Dosing of Anti-C1s Antibody TNT009 – Effect on HLA Antibody-Triggered Complement Activation in Healthy Volunteers and Kidney Transplant Recipients with Antibody-Mediated Rejection. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Muhlbacher J, Jilma B, Wahrmann M, Eskandary F, Gilbert J, Panicker S, Böhmig G. Multiple Dosing of Anti-C1s Antibody TNT009 – Effect on HLA Antibody-Triggered Complement Activation in Healthy Volunteers and Kidney Transplant Recipients with Antibody-Mediated Rejection. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/multiple-dosing-of-anti-c1s-antibody-tnt009-effect-on-hla-antibody-triggered-complement-activation-in-healthy-volunteers-and-kidney-transplant-recipients-with-antibody-mediated-rejection/. Accessed February 20, 2026.« Back to 2017 American Transplant Congress
