Relative Distribution of Livers from HCV+ Donors in the U.S.
1University of California, San Francisco, San Francisco
2Loma Linda University, Loma Linda.
Meeting: 2016 American Transplant Congress
Abstract number: 529
Session Information
Session Name: Concurrent Session: Liver: MELD, Allocation and Donor Issues (DCD/ECD) 2
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:30pm-5:42pm
Presentation Time: 5:30pm-5:42pm
Location: Room 304
Background: HCV-antibody positive donors represent an effective strategy to increase liver availability to HCV+ recipients. However, many HCV+ liver transplant (LT) candidates are now receiving treatment with direct acting antivirals (DAA) that lower the risk of HCV recurrence but could make the patient ineligible for HCV+ donor livers. To better understand the opportunity cost of DAA treatment in HCV+ candidates, we aimed to characterize the relative distribution of HCV+ donor livers in the US.
Methods: We analyzed UNOS data on all US LT waitlist candidates, deceased donor LT recipients, and donors from 7/1/10-6/30/14. Only donor livers that were ultimately transplanted were analyzed.
Results: A total of 868 HCV+ donor livers were utilized during the study period, representing 4% of all deceased donors. The % of deceased donors who were HCV+ varied by region, ranging from 2% in Region 4, 6, 7, and 9 to 6% in Region 2 (Fig). The median (interquartile range) # of offers per HCV+ and HCV- donor livers was 5 (2-17) vs. 3 (1-8) [p<0.01]. The proportion of HCV+ donor livers relative to the 23,131 HCV+ candidates was 4% nationally, ranging from 1% in Region 9 to 7% in Regions 10 and 11 (Fig). Among the 9,356 HCV+ recipients, 9% received an HCV+ liver, which ranged from 4% in Region 6 to 14% in Region 10 (Fig). Median LT-MELD for HCV+ recipients of HCV+ vs. HCV- donor livers was 24 versus 28 (p<0.01).
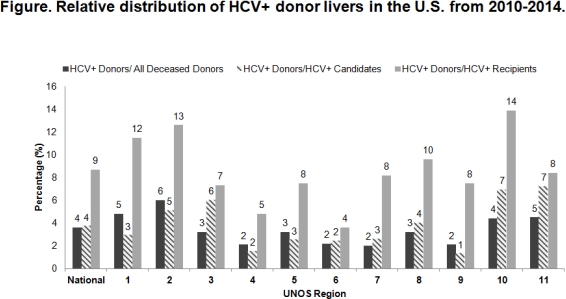
Conclusion: There is significant regional variation in the relative distribution of HCV+ donor livers. The geographic likelihood of receiving an HCV+ donor liver should be taken into account when weighing the risks and benefits of pre-LT HCV treatment. Given the safety and efficacy of HCV treatment and evidence that HCV+ donor livers could allow for earlier transplantation, HCV+ candidates in regions with high HCV+ donor liver availability should consider deferring treatment until after LT.
CITATION INFORMATION: Salazar J, Roberts J, Mehta N, Volk M, Lai J. Relative Distribution of Livers from HCV+ Donors in the U.S. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Salazar J, Roberts J, Mehta N, Volk M, Lai J. Relative Distribution of Livers from HCV+ Donors in the U.S. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/relative-distribution-of-livers-from-hcv-donors-in-the-u-s/. Accessed December 22, 2025.« Back to 2016 American Transplant Congress
