Acute Rejection, New-Onset Diabetes After Transplant and Transplant Outcomes in Pediatric Kidney Recipients: Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) Database.
Nephrology, UCLA, Los Angeles, CA.
Meeting: 2016 American Transplant Congress
Abstract number: D155
Keywords: Kidney, Kidney transplantation, Rejection
Session Information
Session Name: Poster Session D: Kidney-Pediatrics
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
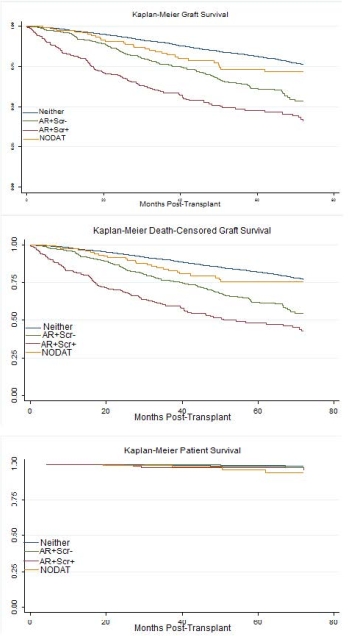
Introduction Our objective was to identify the associations of new-onset diabetes after transplant (NODAT) and acute rejection (AR) in predicting patient mortality and transplant failure within a 6-year follow up time in pediatric kidney recipients.
Methods 4687 patients without preexisting diabetes (age 2-20y, 2004-2010) surviving with a functioning transplant for longer than 1 year with at least one follow up report were identified from the OPTN/UNOS database as of September 2014. Study population was stratified into 4 mutually exclusive groups: patients with a history of AR with serum creatinine levels that returned to baseline after the rejection episode (AR+SCr-), recipients who had a history of AR with elevated serum creatinine level (AR+SCr+) that failed to return to baseline during the follow up time, NODAT, and the reference group (Neither). Multivariate cox regression were used to analyze the relative risks for the outcomes of transplant failure and patient mortality.
Results The median follow-up time was 1827 days after 1-year post-transplant (1397-2533 days, 25th-75th percentiles). NODAT and AR were identified in 3.5% and 14.5% of all study patients, respectively. Hazard ratios of each group are reported in
|
|
Patient Mortality |
|
|
|
Unadjusted HR |
Adjusted HR |
|
Neither |
Reference |
Reference |
|
NODAT |
2.83 (1.02-7.88) |
2.46 (0.88-6.89) |
|
AR+ SCr- |
0.93 (0.33-2.59) |
0.90 (0.32-2.52) |
|
AR+ SCr+ |
2.32 (0.92-5.85) |
2.29 (0.91-5.79) |
and
|
|
Overall Transplant Failure |
Death-Censored Transplant Failure |
||
|
|
Unadjusted HR |
Adjusted HR |
Unadjusted HR |
Adjusted HR |
|
Neither |
Reference |
Reference |
Reference |
Reference |
|
NODAT |
1.39 (0.95-2.03) |
1.16 (0.79-1.70) |
1.30 (0.87-1.94) |
1.08 (0.72-1.61) |
|
AR+ SCr- |
2.33 (1.94-2.80) |
1.92 (1.59-2.32) |
2.38 (1.97-2.87) |
1.95 (1.61-2.36) |
|
AR+ SCr+ |
4.06 (3.31-4.98) |
2.48 (1.99-3.09) |
4.13 (3.36-5.07) |
2.49 (1.99-3.12) |
CITATION INFORMATION: Mehrnia A, Le T, Tamer T, Bunnapradist S. Acute Rejection, New-Onset Diabetes After Transplant and Transplant Outcomes in Pediatric Kidney Recipients: Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) Database. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Mehrnia A, Le T, Tamer T, Bunnapradist S. Acute Rejection, New-Onset Diabetes After Transplant and Transplant Outcomes in Pediatric Kidney Recipients: Analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing (OPTN/UNOS) Database. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/acute-rejection-new-onset-diabetes-after-transplant-and-transplant-outcomes-in-pediatric-kidney-recipients-analysis-of-the-organ-procurement-and-transplant-networkunited-network-for-organ-sharing/. Accessed February 9, 2026.« Back to 2016 American Transplant Congress
