A Change in Insulin Sensitivity and Lipid Profile in Renal Transplant Recipients Converted from Cyclosporine or Standard Release Tacrolimus to Once-Daily Prolonged Release Tacrolimus.
1Internal Medicine, Kosin University College of Medicine, Busan, Republic of Korea
2Urology, Kosin University College of Medicine, Busan, Republic of Korea.
Meeting: 2016 American Transplant Congress
Abstract number: D140
Keywords: Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Agents
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background : New-onset diabetes after transplantation may be associated with the use of tacrolimus (Tac) causing impaired insulin release or reduced insulin sensitivity. And, dyslipidemia commonly occurred after transplantation. Such effects in insulin sensitivity and lipid profile have not been studied in renal transplant recipients receiving traditional twice-daily tacrolimus (TacBID) or cyclosporine and then compared to the new once-daily prolonged release formulation of tacrolimus (TacOD).
Methods : We performed an observational prospective study of 15 stable non-diabetic renal transplant recipients on change in insulin sensitivity and lipid profile in renal transplant recipients converted from cyclosporine or standard release tacrolimus to once-daily prolonged release tacrolimus. We evaluated the level of HbA1c,total cholesterol, HDL, LDL, TG, apolipoprotein A1, apolipoprotein B, serum creatinine, fasting plasma glucose, fasting insulin and HOMA-β at base line, two and four months. To analyze differences in parameter, we performed a t-test in both groups (cyclosporine to TacOD conversion group/TacBID to TacOD conversion group), and GLM-repeated measures ANOVA.
HOMA- β = (360 X Fasting insulin)/(Fasting glucoe-63)
HOMA-IR(insulin resistance) = (Fasting glucose X Fasting insulin)/405
Results : At baseline, parametes were not different in both groups (cyclosporine to TacOD conversion group/TacBID to TacOD conversion group). In GLM-repeated measures ANOVA, the result did not showed and any change in insulin sensitivity and lipid profile after conversion at baseline, two and four months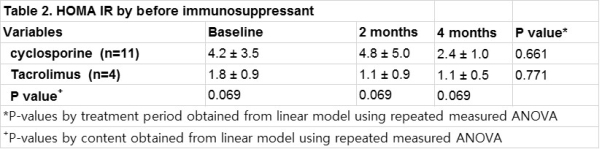 .
.
Conclusion : Conversion from standard TacBID or cyclosporine to TacOD is safe. In spite of a reduced Tac exposure, there was no change in insulin sensitivity and lipid profile in renal transplant recipients.
CITATION INFORMATION: Jung Y, Kim Y, Kim S, Shin H, Rim H, Rhew H. A Change in Insulin Sensitivity and Lipid Profile in Renal Transplant Recipients Converted from Cyclosporine or Standard Release Tacrolimus to Once-Daily Prolonged Release Tacrolimus. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Jung Y, Kim Y, Kim S, Shin H, Rim H, Rhew H. A Change in Insulin Sensitivity and Lipid Profile in Renal Transplant Recipients Converted from Cyclosporine or Standard Release Tacrolimus to Once-Daily Prolonged Release Tacrolimus. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/a-change-in-insulin-sensitivity-and-lipid-profile-in-renal-transplant-recipients-converted-from-cyclosporine-or-standard-release-tacrolimus-to-once-daily-prolonged-release-tacrolimus/. Accessed February 6, 2026.« Back to 2016 American Transplant Congress
