Transplanting Hepatitis C Kidneys into Negative KidnEy Recipients [THINKER] Trial.
University of Pennsylvania, Philadelphia
Meeting: 2017 American Transplant Congress
Abstract number: 1
Keywords: Donation, Hepatitis C, Kidney transplantation
Session Information
Session Time: 8:30am-9:30am
 Presentation Time: 8:30am-8:45am
Presentation Time: 8:30am-8:45am
Location: Arie Crown Theater
Background: More than 500 kidneys from HCV+ deceased donors are discarded each year, even though direct acting antiviral therapies have cure rates exceeding 95%. We performed a pilot trial of transplanting kidneys from HCV+ donors into HCV- recipients (THINKER; NCT02743897; sponsor: Merck).
Methods: HCV- patients aged 40-65 years on dialysis, waitlisted for kidney transplant (KT) with ≤548 days of wait time were approached. A three-step process of education and consent was used pre-enrollment. We performed HCV donor genotyping during allocation, and only used kidneys from genotype 1a or 1b donors. We treated recipients with Grazoprevir/Elbasvir when recipient HCV NAT was detected.
Results: From 6/1/16-11/11/16, 43 patients were contacted by phone, 22 (51.1%) attended an in-person educational session, 19 (86.4%) consented for screening, and 15 (78.9%) were enrolled. 10 HCV- patients received HCV+ kidneys (median KDPI: 42, IQR: 34-53); 9 were genotype 1a. Median time from activation in UNET for HCV+ donors and KT was 58 days (range 11-130 days). All 10 patients had detectable HCV RNA on post-op day 3, but were undetectable within 4 weeks of starting HCV therapy. As of 12/1/2016, 6 patients completed 12 weeks of HCV treatment[mdash]one had sustained virologic response (SVR)-12 ('cure'), two had SVR-6, two had SVR-4, and one had SVR-2, while the other four had undetectable virus on treatment. All had excellent allograft function.
As of 12/1/2016, 6 patients completed 12 weeks of HCV treatment[mdash]one had sustained virologic response (SVR)-12 ('cure'), two had SVR-6, two had SVR-4, and one had SVR-2, while the other four had undetectable virus on treatment. All had excellent allograft function. 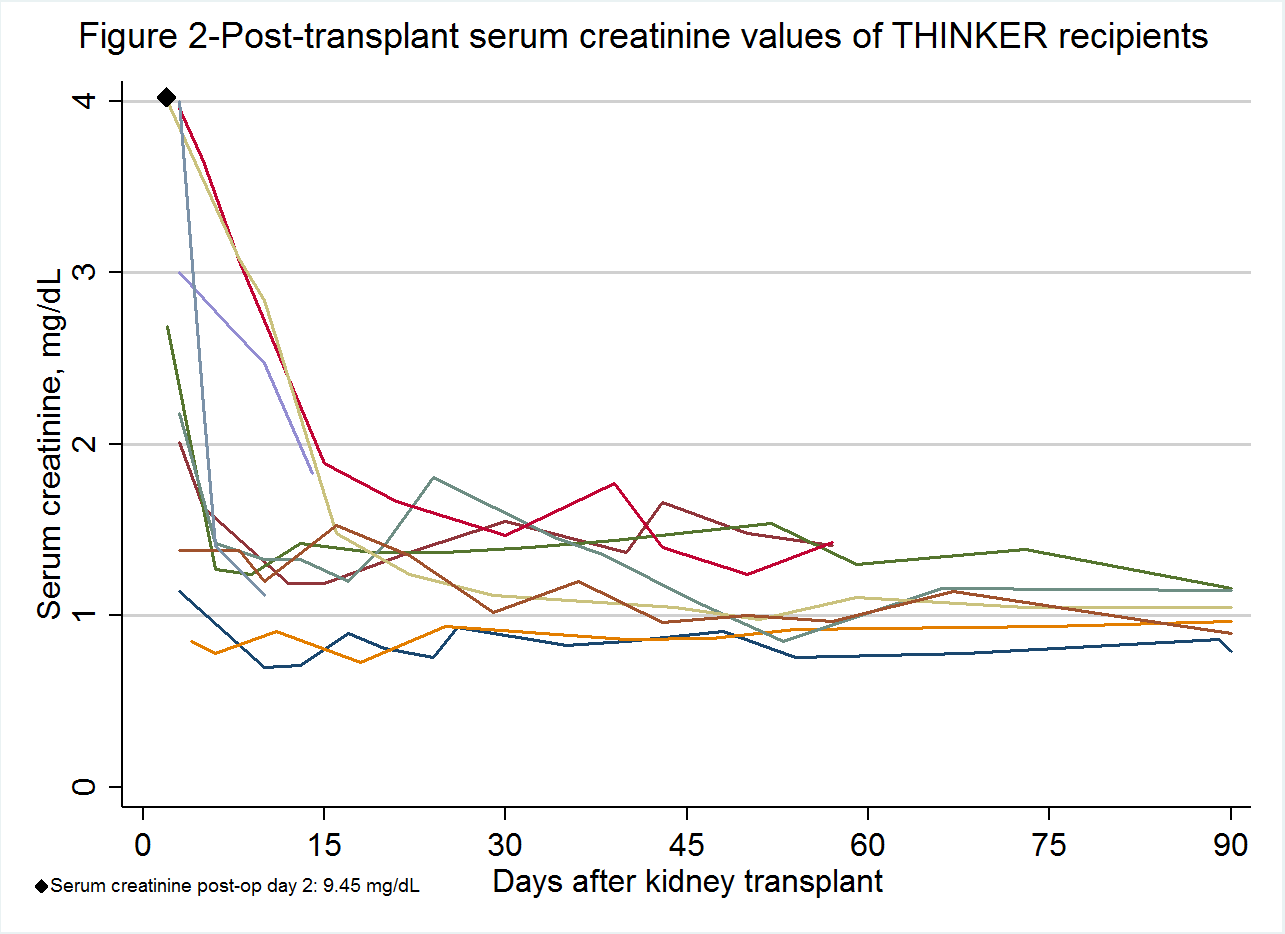 There were no adverse events related to HCV transmission or treatment.
There were no adverse events related to HCV transmission or treatment.
Conclusions: In this pilot trial, we demonstrate safety and efficacy of transplanting kidneys from HCV+ donors into HCV- patients. Future work is needed in a larger sample, but these results suggest that HCV+ kidneys may be valuable to a wide range of candidates.
CITATION INFORMATION: Goldberg D, Blumberg E, Reddy R, Nazarian S, Van Deerlin V, Trofe-Clarke J, Levine M, Sawinski D, Bloom R, Abt P, Reese P. Transplanting Hepatitis C Kidneys into Negative KidnEy Recipients [THINKER] Trial. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Goldberg D, Blumberg E, Reddy R, Nazarian S, Deerlin VVan, Trofe-Clarke J, Levine M, Sawinski D, Bloom R, Abt P, Reese P. Transplanting Hepatitis C Kidneys into Negative KidnEy Recipients [THINKER] Trial. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/transplanting-hepatitis-c-kidneys-into-negative-kidney-recipients-thinker-trial/. Accessed December 10, 2025.« Back to 2017 American Transplant Congress
